推薦產品
形狀
solid
mp
123-126 °C (lit.)
SMILES 字串
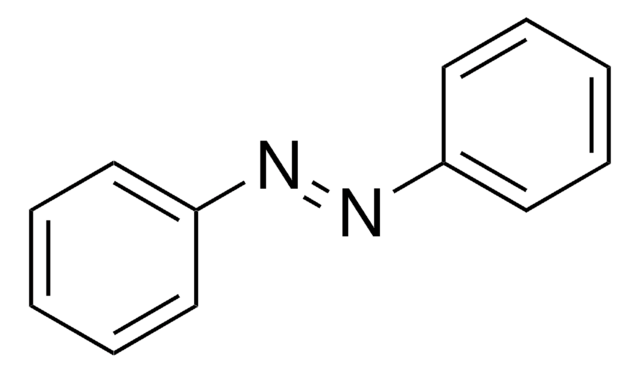
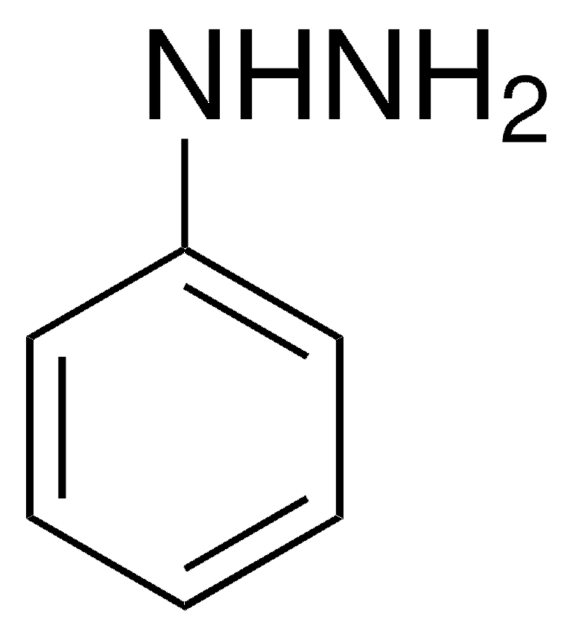
N(Nc1ccccc1)c2ccccc2
InChI
1S/C12H12N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10,13-14H
InChI 密鑰
YBQZXXMEJHZYMB-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
氢化偶氮苯是氧化偶氮苯氢化成苯胺的中间体。
通过在 THF 中用 SmI(2)还原偶氮苯来制备氢化偶氮苯。
應用
作为以下反应的反应物:
- 与有机金属钽配合物发生的插入反应
- 三氯化钛(III)催化的还原反应,生成胺
- 研究苯重排的机理
- 与 N-杂环稳定的甲硅烷基发生的反应
- 二锰酰胺酰肼簇合物的合成
- 铁介导的肼还原反应,生成亚芳基铁立方烷
其他說明
含有不定量的偶氮苯
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
Chintada Nageswara Rao et al.
The Journal of organic chemistry, 76(22), 9438-9443 (2011-10-19)
The reduction of azobenzene by SmI(2) in THF to give hydrazobenzene was investigated. The kinetics are first order in the substrate and first order in SmI(2). The kinetic order in MeOH is ca. 0.56, and in TFE it is ca.
Darol E Dodd et al.
International journal of toxicology, 31(6), 564-571 (2012-11-09)
Male F344 rats were exposed to hydrazobenzene (HZB) by dietary feed at concentrations of 0, 5, 20, 80, 200, or 300 ppm for 5 days, 2 weeks, 4 weeks, or 13 weeks duration. End points evaluated included clinical observations, body
Hydrazobenzene.
Report on carcinogens : carcinogen profiles, 10, 139-140 (2004-08-26)
H Fabre et al.
Journal of pharmaceutical sciences, 73(12), 1706-1709 (1984-12-01)
A high-performance liquid chromatographic method was developed for the simultaneous determination of azobenzene, hydrazobenzene, and four other decomposition products in phenylbutazone injectable formulations. Separation was achieved on a C18 column, with 0.1 M Tris-citrate buffer (pH 5.25) and acetonitrile (52:48)
Jenny V Lockard et al.
The journal of physical chemistry. A, 109(6), 1205-1215 (2006-07-13)
A quantitative model of mixed-valence excited-state spectroscopy is developed and applied to 2,3-diphenyl-2,3-diazabicyclo[2.2.2]octane. The lowest-energy excited state of this molecule arises from a transition from the ground state, where the charge is located on the hydrazine bridge, to an excited
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務