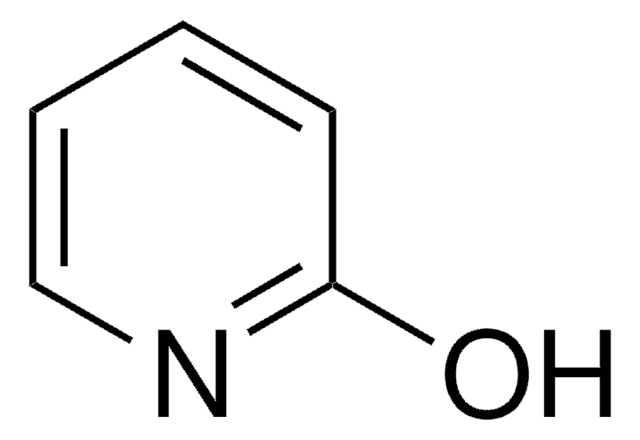
推薦產品
品質等級
化驗
95%
bp
230-235 °C/12 mmHg (lit.)
mp
150-151 °C (lit.)
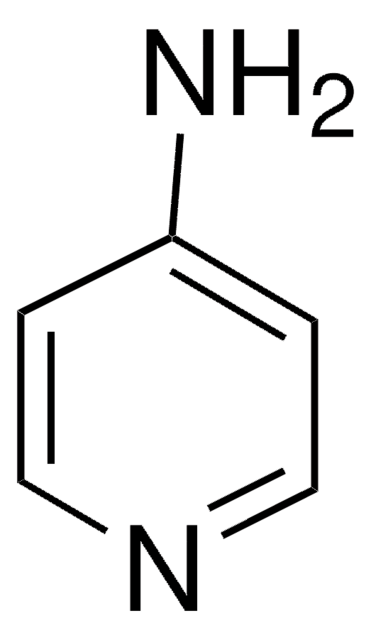
SMILES 字串
O=C1C=CNC=C1
InChI
1S/C5H5NO/c7-5-1-3-6-4-2-5/h1-4H,(H,6,7)
InChI 密鑰
GCNTZFIIOFTKIY-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
4-羟基吡啶被用于通过水(溶剂化)热方法合成(Ag3MoO3F3) (Ag3MoO4)Cl。它被用作模型化合物来研究代表性水生环境污染物的自然光降解。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Natsuki K Kubota et al.
Bioorganic & medicinal chemistry, 11(21), 4569-4575 (2003-10-07)
Piericidins C5 (1) and C6 (2), two new members of the piericidin family, were isolated from a Streptomyces sp. and a Nocardioides sp., together with known piericidins C1 (3), C2 (4), C3 (5), C4 (6), D1 (7), and A3 (8).
Xiaoqing Cai et al.
Bioorganic & medicinal chemistry, 20(11), 3584-3595 (2012-05-09)
Bicyclic pyridinol antioxidants have been reported to suppress the autoxidation of methyl linoleate more effectively than α-tocopherol in benzene solution. A few novel lipophilic analogues have recently been synthesized by conjugating a pyridinol core with the phytyl side chain of
Alignment of acentric MoO3F33-anions in a polar material :(AgMoO3F3)(Ag3MoO4) Cl.
Maggard PA, et al.
Journal of Solid State Chemistry, 175(1), 27-33 (2003)
Heiko Zettl et al.
ACS chemical biology, 7(9), 1488-1495 (2012-06-26)
We present an integrated approach to identify and optimize a novel class of γ-secretase modulators (GSMs) with a unique pharmacological profile. Our strategy included (i) virtual screening through application of a recently developed protocol (PhAST), (ii) synthetic chemistry to discover
H Chen et al.
Biochemistry, 32(43), 11591-11599 (1993-11-02)
We have examined the interaction of Citrobacter freundii tyrosine phenol-lyase with both L- and D-alanine. This enzyme catalyzes the racemization of alanine as a side reaction, in addition to the physiological beta-elimination of L-tyrosine to give phenol and ammonium pyruvate.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務