推薦產品
化驗
98%
mp
161-163 °C (lit.)
溶解度
methanol: soluble
官能基
carboxylic acid
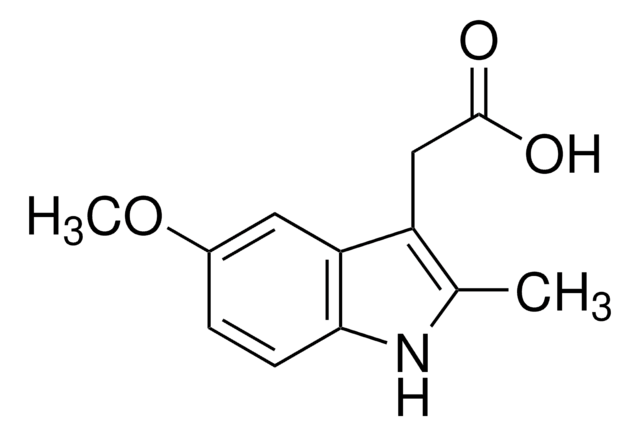
SMILES 字串
COc1ccc2[nH]c(C)c(CC(O)=O)c2c1
InChI
1S/C12H13NO3/c1-7-9(6-12(14)15)10-5-8(16-2)3-4-11(10)13-7/h3-5,13H,6H2,1-2H3,(H,14,15)
InChI 密鑰
TXWGINUZLBAKDF-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
5-Methoxy-2-methyl-3-indoleacetic acid is hydrolysis product of indomethacin.
應用
5-Methoxy-2-methyl-3-indoleacetic acid was used for quantitative determination of indomethacin and its major impurities in suppository and capsule formulations by HPLC. 5-Methoxy-2-methyl-3-indoleacetic acid was used in a study to develop fast, sensitive and simultaneous determination of metabolites of serotonin using liquid chromatography with mass spectrometric detection.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
W I Rosenblum et al.
Prostaglandins, 21(4), 667-673 (1981-04-01)
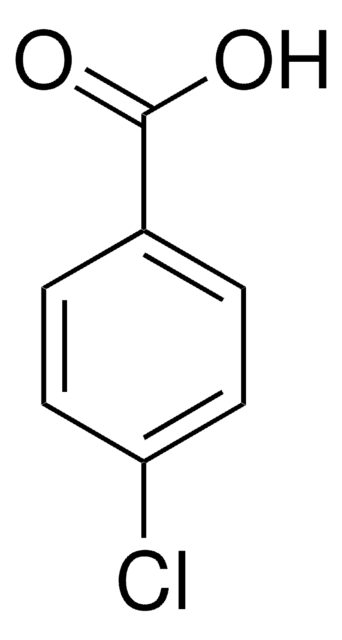
Previous studies suggested that one of the hydrolysis products of indomethacin, either 4-chlorobenzoic acid or 5-methoxy-2-methyl-3-indole acetic acid, can inhibit platelet aggregation in vivo. If correct, this hypothesis explains the apparent action of indomethacin dissolved at high pH where hydrolysis
T B Vree et al.
Journal of chromatography, 616(2), 271-282 (1993-07-02)
Indomethacin is metabolized in humans by O-demethylation, and by acyl glucuronidation to the 1-O-glucuronide. Indomethacin, its metabolite O-desmethylindomethacin (DMI) and their conjugates can be measured directly by gradient high-performance liquid chromatographic analysis without enzymic deglucuronidation. The glucuronide conjugates were isolated
H Alho et al.
Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research, 5(9), 1005-1014 (1994-09-01)
A recognition site for benzodiazepines structurally different from that linked to the gamma-aminobutyric acid receptor subtype A or the "central type" benzodiazepine receptor has been located mainly in the outer membranes of mitochondria and designated mitochondrial benzodiazepine receptor (MBR). A
Peng Zhang et al.
Acta pharmacologica Sinica, 27(8), 1097-1102 (2006-07-27)
To investigate the biotransformation of indomethacin, the first of the newer nonsteroidal anti-inflammatory drugs, by filamentous fungus and to compare the similarities between microbial transformation and mammalian metabolism of indomethacin. Five strains of Cunninghamella (C elegans AS 3.156, C elegans
E Kwong et al.
Journal of pharmaceutical sciences, 71(7), 828-830 (1982-07-01)
Indomethacin and its impurities in suppository and capsule formulations were quantitatively determined by HPLC using a reversed-phase, octadecyl column and a mobile phase of methanol-water-acetonitrile-acetic acid (55:35:10:1). Analysis of the suppository formulations provided a mean potency for indomethacin of 103.8%.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務