51086
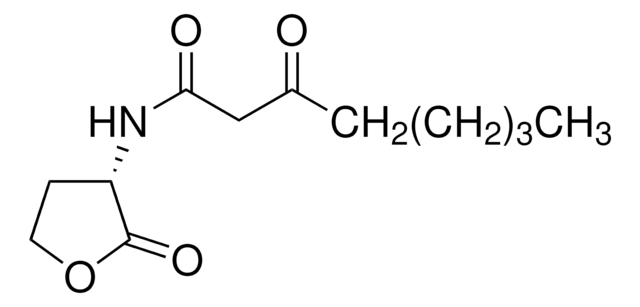
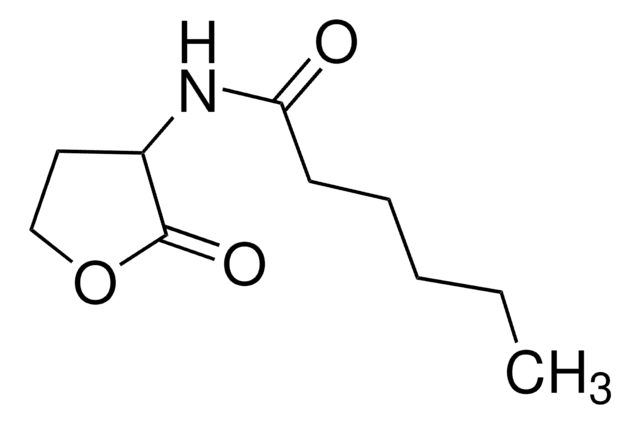
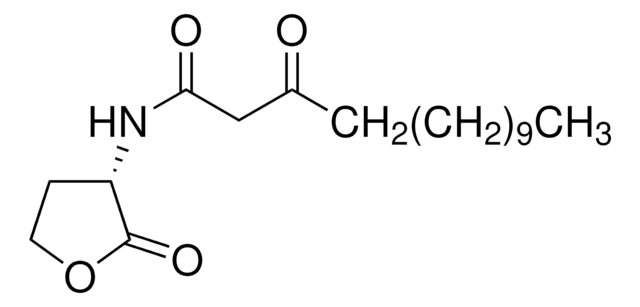
N-Heptanoyl-L-homoserine lactone
≥96% (HPLC)
Synonym(s):
N-[(3S)-Tetrahydro-2-oxo-3-furanyl]heptanamide, C7-HSL
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H19NO3
CAS Number:
Molecular Weight:
213.27
Beilstein:
10258594
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.85
Recommended Products
Product Name
N-Heptanoyl-L-homoserine lactone, ≥96% (HPLC)
Quality Level
Assay
≥96% (HPLC)
form
powder or crystals
optical activity
[α]/D -29±3°, c = 0.2 in methanol
color
white
suitability
conforms to structure for Proton NMR spectrum
storage temp.
−20°C
SMILES string
O=C1OCC[C@@H]1NC(CCCCCC)=O
InChI
1S/C11H19NO3/c1-2-3-4-5-6-10(13)12-9-7-8-15-11(9)14/h9H,2-8H2,1H3,(H,12,13)/t9-/m0/s1
InChI key
FTMZLSDESAOPSZ-VIFPVBQESA-N
Biochem/physiol Actions
N-Heptanoyl-L-homoserine lactone is a member of N-acyl-homoserine lactone family. N-Acylhomoserine lactones (AHL) regulate gene expression in gram-negative bacteria, such as Echerichia and Salmonella, and are involved in quorum sensing, cell to cell communication among bacteria; for reviews see. Bacterial intercellular communication has become a target for the development of new anti-virulence drugs, and a research focus for the prevention of biofilm formation.
Quorum-sensing signal generation
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J K Lithgow et al.
Molecular microbiology, 37(1), 81-97 (2000-08-10)
N-(3-hydroxy-7-cis-tetradecenoyl)-L-homoserine lactone (3OH, C14:1-HSL) is a quorum-sensing signalling molecule produced by Rhizobium leguminosarum. It is unusual in that it inhibits the growth of several strains of R. leguminosarum and was previously known as 'small bacteriocin'. The cinRI locus responsible for
F Wisniewski-Dyé et al.
Journal of bacteriology, 184(6), 1597-1606 (2002-03-02)
Analysis of N-acyl-L-homoserine lactones (AHLs) produced by Rhizobium leguminosarum bv. viciae indicated that there may be a network of quorum-sensing regulatory systems producing multiple AHLs in this species. Using a strain lacking a symbiosis plasmid, which carries some of the
Wai-Fong Yin et al.
Sensors (Basel, Switzerland), 12(3), 3472-3483 (2012-06-28)
Bacteria communicate by producing quorum sensing molecules called autoinducers, which include autoinducer-1, an N-hexanoyl homoserine lactone (AHL), and autoinducer-2. Bacteria present in the human oral cavity have been shown to produce autoinducer-2, but not AHL. Here, we report the isolation
Megan E Pomianek et al.
ACS chemical biology, 2(5), 293-295 (2007-05-24)
Small-molecule agonists and antagonists of bacterial quorum sensing can enhance our understanding of this form of cell-cell communication. A recent effort has discovered effective modulators of the autoinducer-1 circuit for bacterial quorum sensing by the synthesis and evaluation of a
Kaimin Niu et al.
Indian journal of microbiology, 57(3), 329-338 (2017-09-15)
An increasing concern on resistance to multiple-antibiotics has led to the discovery of novel agents and the establishment of new precaution strategy. Numerous plant sources have been widely studied to reduce virulence of pathogenic bacteria by interfering cell-to-cell based communication
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service