O9139
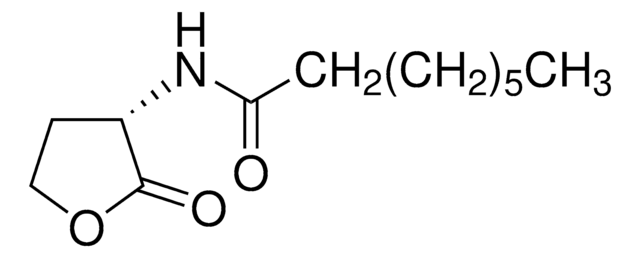
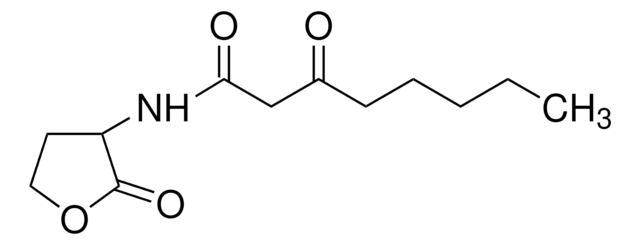
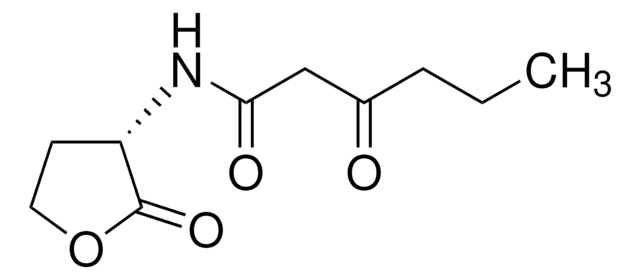
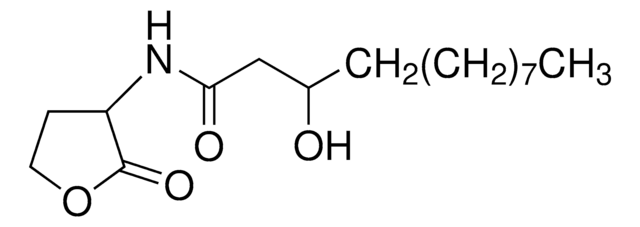
N-(3-Oxododecanoyl)-L-homoserine lactone
≥98% (TLC)
Synonym(s):
3-Oxo-C12-HSL
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C16H27NO4
CAS Number:
Molecular Weight:
297.39
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
N-(3-Oxododecanoyl)-L-homoserine lactone, quorum sensing signaling molecule
Assay
≥98% (TLC)
form
powder
color
white
application(s)
cell analysis
shipped in
wet ice
storage temp.
−20°C
SMILES string
CCCCCCCCCC(=O)CC(=O)N[C@H]1CCOC1=O
InChI
1S/C16H27NO4/c1-2-3-4-5-6-7-8-9-13(18)12-15(19)17-14-10-11-21-16(14)20/h14H,2-12H2,1H3,(H,17,19)/t14-/m0/s1
InChI key
PHSRRHGYXQCRPU-AWEZNQCLSA-N
Application
N-(3-Oxododecanoyl)-L-homoserine lactone (3-Oxo-C12-HSL), a member of a family of acyl homoserine lactones, may be used to determine its mechanisms and specificity of action as a signaling molecule involved in the regulation of bacterial quorum sensing.
Biochem/physiol Actions
N-(3-oxododecanoyl)homoserine-L-lactone (3-oxo-C12-HSL) is among a group of homoserine lactones that includes: N-octanoyl-homoserine lactone (N-C8-HSL), N-(3-oxodecanoyl) homoserine-L-lactone (3-oxo-C10 HSL), N-(3-Oxotetradecanoyl)-L-homoserine lactone (3-oxo-C14-HSL, N-(3-hydroxydecanoyl)-L-homoserine lactone, and N-(3-hydroxyoctanoyl)-L-homoserine lactone involved in the processes of bacterial quorum sensing. These N-acyl-homoserine lactones are used to study the processes and mechanisms of bacterial quorum sensing.
Acyl homoserine lactone that is an autoinducer of quorum sensing by Pseudomonas aeruginosa. Degraded by paraoxonase (PON) family members.
also commonly purchased with this product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Robust multicellular computing using genetically encoded NOR gates and chemical `wires?
Tamsir A, et al.
Nature, 469(7329), 212-215 (2011)
Kaihao Tang et al.
Scientific reports, 3, 2935-2935 (2013-10-15)
Quorum sensing (QS) is a population-dependent mechanism for bacteria to synchronize social behaviors such as secretion of virulence factors. The enzymatic interruption of QS, termed quorum quenching (QQ), has been suggested as a promising alternative anti-virulence approach. In order to
Yunhui Zhang et al.
BMC genomics, 16, 38-38 (2015-02-06)
Intestinal microbes play significant roles in fish and can be possibly used as probiotics in aquaculture. In our previous study, Flaviramulus ichthyoenteri Th78(T), a novel species in the family Flavobacteriaceae, was isolated from fish intestine and showed strong quorum quenching
Chihiro Ueda et al.
Antimicrobial agents and chemotherapy, 54(2), 683-688 (2009-11-18)
We have examined the potential bactericidal activities of several tetramic acids derived from Pseudomonas autoinducers against Clostridium difficile, a cause of antibiotic-associated pseudomembranous colitis. Clinical isolates of C. difficile (n=4) were incubated in broth with a chemically synthesized Pseudomonas autoinducer
Gene PA2449 Is Essential for Glycine Metabolism and Pyocyanin
Biosynthesis in Pseudomonas aeruginosa PAO1
Biosynthesis in Pseudomonas aeruginosa PAO1
Lundgren BR, et al.
Journal of Bacteriology, 195(9), 2087-2100 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service