T19526
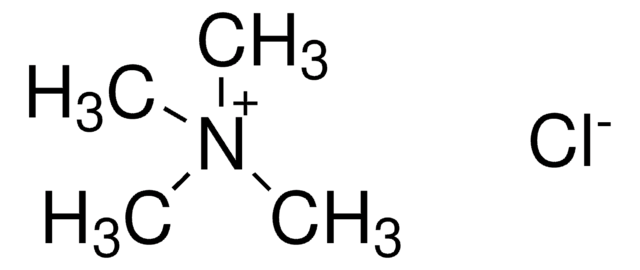
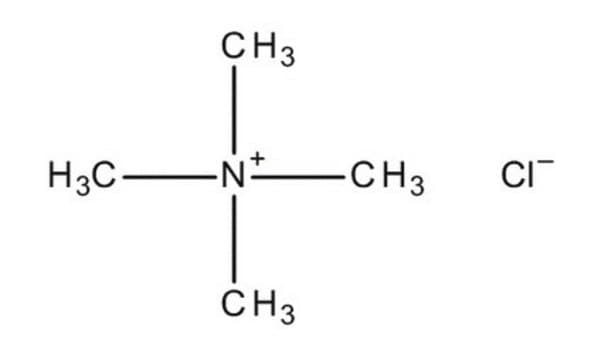
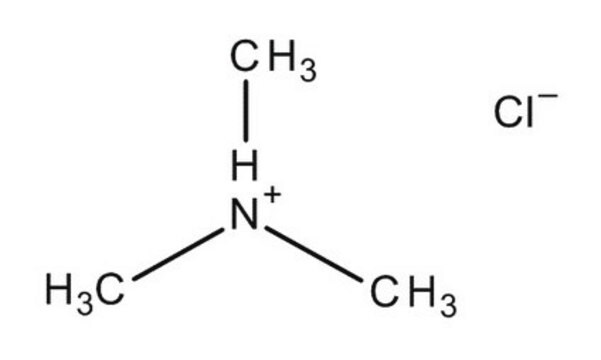
Tetramethylammonium chloride
reagent grade, ≥98%
Synonym(s):
TMA
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
(CH3)4N(Cl)
CAS Number:
Molecular Weight:
109.60
Beilstein:
3911201
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
reagent grade
Quality Level
Assay
≥98%
mp
>300 °C (lit.)
functional group
amine
SMILES string
[Cl-].C[N+](C)(C)C
InChI
1S/C4H12N.ClH/c1-5(2,3)4;/h1-4H3;1H/q+1;/p-1
InChI key
OKIZCWYLBDKLSU-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tetramethylammonium chloride is a quaternary ammonium salt commonly used as a catalyst due to its thermal stability and also tolerance towards strong aqueous bases or nucleophiles.
Application
Tetramethylammonium chloride along with N-hydroxyphthalimide and xanthone may be used as an efficient chloride catalytic system for the aerobic oxidation of hydrocarbons to form the corresponding oxygenated compounds. It may also be used as a phase transfer catalyst for the synthesis of aryl fluorides via selective chloride/fluoride exchange reaction of activated aryl chlorides with potassium fluoride in solid-liquid phase.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Aquatic Chronic 2 - Skin Irrit. 2 - STOT SE 1 Oral
Target Organs
Central nervous system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A free radical process for oxidation of hydrocarbons promoted by nonmetal xanthone and tetramethylammonium chloride under mild conditions.
Du Z, et al.
Tetrahedron Letters, 50(15), 1677-1680 (2009)
Tetramethylammonium chloride as a selective and robust phase transfer catalyst in a solid?liquid halex reaction: the role of water.
Sasson Y, et al.
Chemical Communications (Cambridge, England), 3, 297-298 (1996)
Min Bum Park et al.
Journal of the American Chemical Society, 135(6), 2248-2255 (2012-11-29)
A solid understanding of the molecular-level mechanisms responsible for zeolite crystallization remains one of the most challenging issues in modern zeolite science. Here we investigated the formation pathway for high-silica LTA zeolite crystals in the simultaneous presence of tetraethylammonium (TEA(+))
Seonki Hong et al.
Science advances, 4(9), eaat7457-eaat7457 (2018-09-12)
Biological functions depend on biomolecular assembly processes. Assemblies of lipid bilayers, actins, microtubules, or chromosomes are indispensable for cellular functions. These hierarchical assembly processes are reasonably predictable by understanding chemical structures of the defined building blocks and their interactions. However
Markus Welcker et al.
Genes & development, 27(23), 2531-2536 (2013-12-04)
The Fbw7 tumor suppressor targets a broad network of proteins for ubiquitylation. Here we show critical functions for Fbw7 dimerization in regulating the specificity and robustness of degradation. Dimerization enables Fbw7 to target substrates through concerted binding to two suboptimal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service