208264
Allyltrimethylsilane
98%
Synonym(s):
3-(Trimethylsilyl)propene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
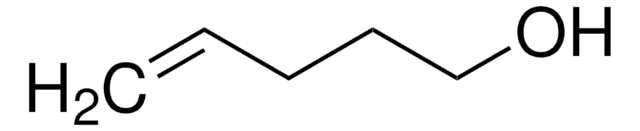
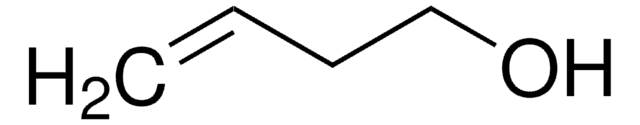
Linear Formula:
H2C=CHCH2Si(CH3)3
CAS Number:
Molecular Weight:
114.26
Beilstein:
906755
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.407 (lit.)
bp
84-88 °C (lit.)
density
0.719 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)CC=C
InChI
1S/C6H14Si/c1-5-6-7(2,3)4/h5H,1,6H2,2-4H3
InChI key
HYWCXWRMUZYRPH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Allyltrimethylsilane is a general reagent to introduce allyl groups across acid chlorides, aldehydes, ketones, iminium ions, enones, and for cross-coupling with other carbon electrophiles. It is used as a reagent in Hosomi−Sakurai reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Allyltrimethylsilane
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2001)
Armando Ramirez et al.
Organic letters, 7(21), 4617-4620 (2005-10-08)
[reaction: see text] The annulation reactions of alkenes with peroxycarbenium ions enable the synthesis of a variety of functionalizable 1,2-dioxolanes. Triethysilyl-protected peroxycarbenium ions proved to be optimal for the annulation reaction. Using this method, plakinic acid analogues can be synthesized
Allylation of Imines with Allyltrimethylsilane and Experimental Evidences for a Fluoride-Triggered Autocatalysis Mechanism of the Sakurai- Hosomi Reaction
Wang D, et al.
The Journal of Organic Chemistry, 64(12), 4233-4237 (1999)
Shoji Kobayashi et al.
Carbohydrate research, 343(3), 443-452 (2007-12-11)
An efficient route to the trans-fused tetrahydrooxepin corresponding to the E ring of ciguatoxin was developed. Wide screening of allylation reactions of sulfur or fluoro-substituted tetrahydrooxepin revealed that the optimum method for obtaining the beta-allylation product selectively was the use
Allyltrimethylsilane
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Articles
Reagents for C–C Bond Formation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service