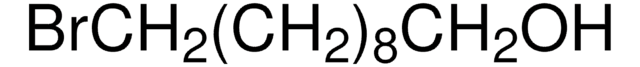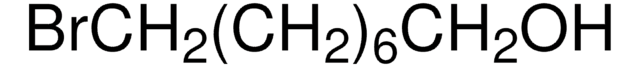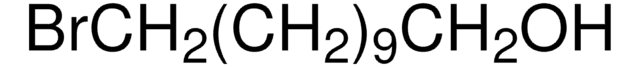167169
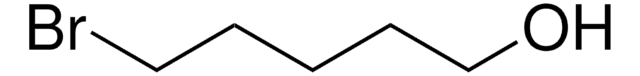
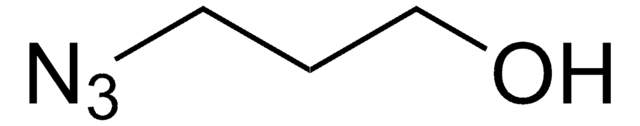
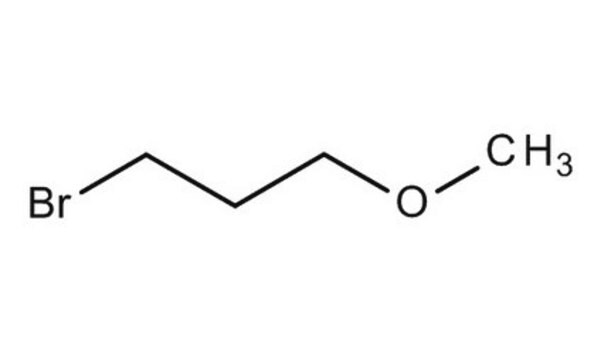
3-Bromo-1-propanol
97%
Synonym(s):
Trimethylene bromohydrin
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Br(CH2)3OH
CAS Number:
Molecular Weight:
138.99
Beilstein:
969160
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.488 (lit.)
bp
62 °C/5 mmHg (lit.)
density
1.537 g/mL at 25 °C (lit.)
functional group
bromo
storage temp.
2-8°C
SMILES string
OCCCBr
InChI
1S/C3H7BrO/c4-2-1-3-5/h5H,1-3H2
InChI key
RQFUZUMFPRMVDX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Reaction between 3-bromo-1-propanol, phenol and a series of phenols having substituents in 4-position has been studied in micellar media and in microemulsions based on cationic or a nonionic surfactant.
3-Bromo-1-propanol is an electrophile used as a substrate in nucleophilic substitution reactions and in the redox polymerization.
3-Bromo-1-propanol is an electrophile used as a substrate in nucleophilic substitution reactions and in the redox polymerization.
Application
3-Bromo-1-propanol was used in the synthesis of fluorescent halide-sensitive quinolinium dyes and molten salt-polymers. It was used in the synthesis of chiral, quaternary prolines via cyclization of quaternary amino acids.
Recently used as a grafting agent in the synthesis of recyclable reagents for Swern oxidation
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
149.0 °F - closed cup
Flash Point(C)
65 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fredrik Currie
Journal of colloid and interface science, 277(1), 230-234 (2004-07-28)
The reaction between 3-bromo-1-propanol and phenol and a series of phenols carrying substituents in 4-position was studied in micellar media and in microemulsions based on either a cationic or a nonionic surfactant. The reactivity and the yield were evaluated and
Takeo Kawabata et al.
Journal of the American Chemical Society, 128(48), 15394-15395 (2006-11-30)
An enantiodivergent asymmetric cyclization of N-Boc-N-omega-bromoalkyl-alpha-amino acid derivatives has been developed. With potassium amide bases in DMF, cyclization proceeds with retention of configuration, while inversion of configuration was observed with lithium amide bases in THF. Chirality of the parent amino
Wei Song et al.
Molecules (Basel, Switzerland), 25(17) (2020-08-23)
Azobenzene (AB) units were successfully introduced into poly(1,6-heptadiyne)s in order to ensure smooth synthesis of double- and single-stranded poly(1,6-heptadiyne)s (P1 and P2) and simultaneously realize the self-assembly by Grubbs-III catalyst-mediated metathesis cyclopolymerization (CP) of AB-functionalized bis(1,6-heptadiyne) and 1,6-heptadiyne monomers (M1
Synthesis of molten salt-type polymer brush and effect of brush structure on the ionic conductivity.
Yoshizawa M and Ohno H.
Electrochimica Acta, 46(10), 1723-1728 (2001)
C D Geddes et al.
Analytical biochemistry, 293(1), 60-66 (2001-05-25)
Three fluorescent halide-sensitive quinolinium dyes have been produced by the reaction of the 6-methylquinoline heterocyclic nitrogen base with methyl bromide, methyl iodide, and 3-bromo-1-propanol. The quaternary salts, unlike the precursor molecule, are readily water soluble and the fluorescence intensity of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service