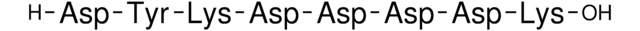CELLMM2
FLAG® M Purification Kit
For Mammalian expression systems.
Synonym(s):
Anti-ddddk, Anti-dykddddk
About This Item
Recommended Products
Application
Learn more product details in our FLAG® application portal.
Features and Benefits
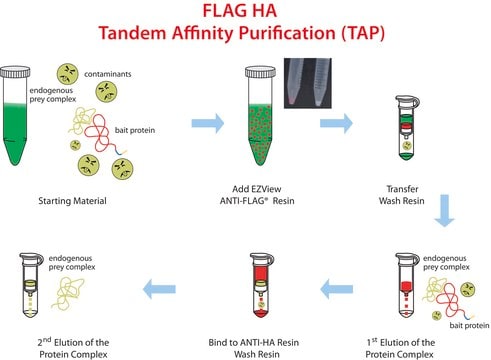
- Includes ready to use reagents, columns, and a detailed protocol for affinity purification of FLAG fusion proteins.
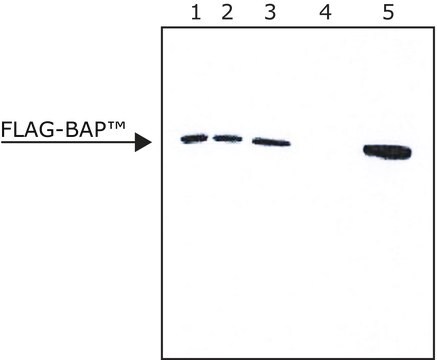
- ANTI-FLAG M2 Affinity Gel allows efficient binding of FLAG fusion proteins without the need for preliminary steps and calibrations.
- Two alternatives for efficient elution conditions (by acidic conditions or by competition with FLAG peptide).
Packaging
Other Notes
Legal Information
Kit Components Also Available Separately
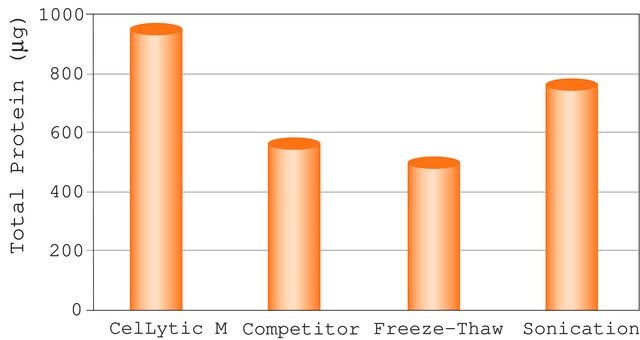
- C2978CelLytic™ M, Cell Lysis Reagent, Suitable for Mammalian cell lysis and protein solubilization.SDS
- SAE0194Purified 3xFLAG® peptide, ≥95% (HPLC), lyophilized powderSDS
- A2220ANTI-FLAG® M2 Affinity Gel, purified immunoglobulin, buffered aqueous glycerol solutionSDS
- C2103Chromatography columns, general-purpose, volume 10 mL, Overall H 5 in.SDS
Storage Class Code
10 - Combustible liquids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Protein purification techniques, reagents, and protocols for purifying recombinant proteins using methods including, ion-exchange, size-exclusion, and protein affinity chromatography.
Protein expression technologies for expressing recombinant proteins in E. coli, insect, yeast, and mammalian expression systems for fundamental research and the support of therapeutics and vaccine production.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service