M29800
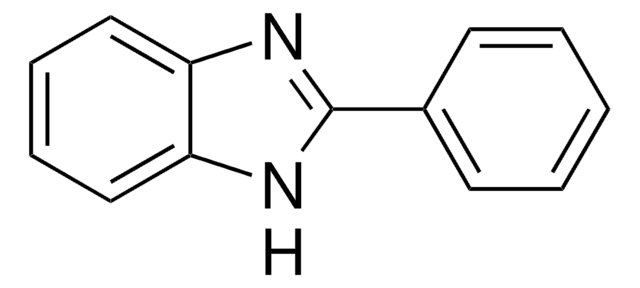
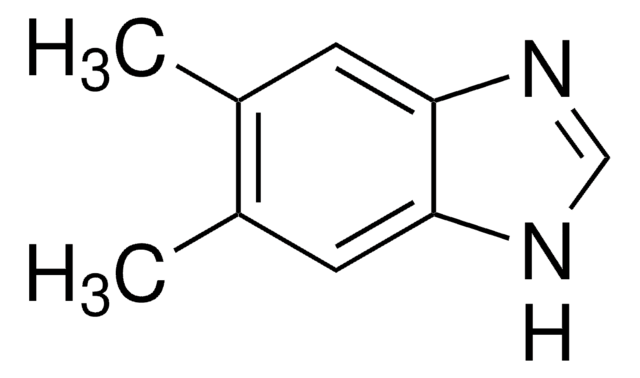
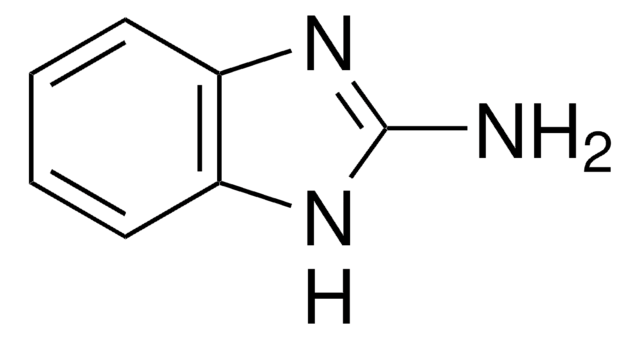
2-Methylbenzimidazole
98%
Synonym(s):
2-Methyl-1H-1,3-benzodiazole, 2-Methyl-1H-benzimidazole, 2-Methyl-1H-benzo[d]imidazole, 2-Methylbenzoimidazole
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H8N2
CAS Number:
Molecular Weight:
132.16
Beilstein:
112264
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
175-177 °C (lit.)
SMILES string
Cc1nc2ccccc2[nH]1
InChI
1S/C8H8N2/c1-6-9-7-4-2-3-5-8(7)10-6/h2-5H,1H3,(H,9,10)
InChI key
LDZYRENCLPUXAX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- 2-Methylbenzimidazole is an important pharmacophore widely used in medicinal chemistry for the synthesis of various antibacterial and antifungal agents.
- It can be used as a key precursor to synthesize substituted benzimidazo[1,2-a]quinolones.
- It can be used in the synthesis of reversible solid-to-liquid phase transition coordination polymer crystals.
- 2-Methylbenzimidazole also exhibits corrosion inhibition.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and biological evaluation of substituted benzimidazoles.
Shah K, et al.
Journal of the Indian Chemical Society, 93, 1009-1018 (2016)
Deidré van den Berg et al.
Bioorganic & medicinal chemistry, 15(11), 3692-3702 (2007-04-10)
We have recently reported that a series of (E)-8-styrylcaffeines and (E)-2-styrylbenzimidazoles are moderate to very potent competitive inhibitors of monoamine oxidase B (MAO-B). The most potent member of the series was found to be (E)-8-(3-chlorostyryl)caffeine (CSC) with an enzyme-inhibitor dissociation
Reversible solid-to-liquid phase transition of coordination polymer crystals.
Umeyama D, et al.
Journal of the American Chemical Society, 137(2), 864-870 (2015)
Synthesis and Antimicrobial Activity of Some Benzimidazole and 2-Methylbenzimidazole Derivatives.
Jain P and Tiwari M
Asian Journal of Chemistry, 29(4), 838-838 (2017)
Novel strategy for synthesis of substituted benzimidazo [1, 2-a] quinolines.
Kato J Y, et al.
Organic Letters, 15(14), 3794-3797 (2013)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| M29800-100G | 4061838354990 |
| M29800-5G | 4061834046233 |
| M29800-5KG |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service