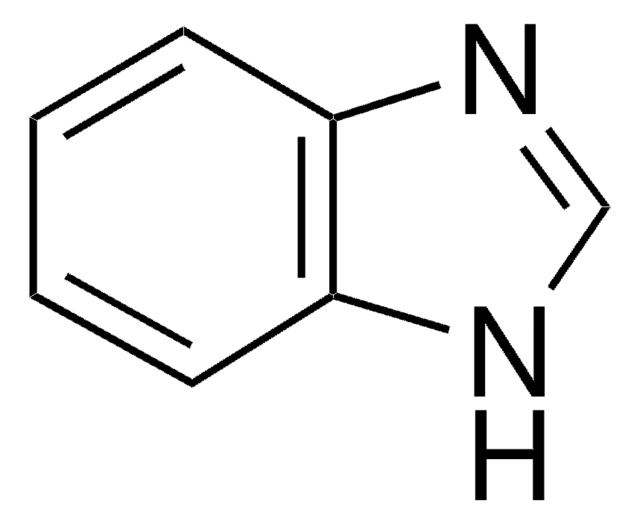
399353
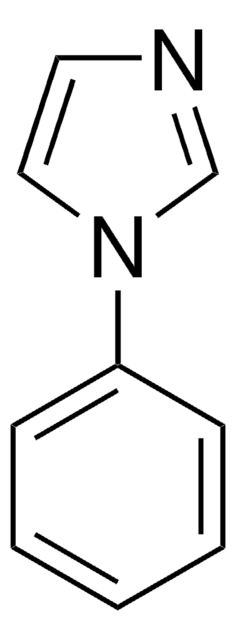
1-Methylbenzimidazole
99%
Synonym(s):
1-Methyl-1H-benzimidazole, 1-Methyl-1H-benzo[d]imidazole, 3-Methylbenzimidazole, N-Methylbenzimidazole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H8N2
CAS Number:
Molecular Weight:
132.16
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
154 °C/12 mmHg (lit.)
mp
59-62 °C (lit.)
SMILES string
Cn1cnc2ccccc12
InChI
1S/C8H8N2/c1-10-6-9-7-4-2-3-5-8(7)10/h2-6H,1H3
InChI key
FGYADSCZTQOAFK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Methylbenzimidazole is a heterocyclic building block. Effect of the interaction between 1-methylbenzimidazole (MBI) with Li+ and TiO2 on the performance of dye-sensitized solar cells has been investigated.
Application
1-Methylbenzimidazole is the suitable reagent used as electrolyte additive for the dye-sensitized solar cells and solid-state dye-sensitized solar cells.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hlaing H Maw et al.
Drug metabolism and disposition: the biological fate of chemicals, 46(6), 770-778 (2018-03-09)
BI 187004, an 11β-hydroxysteroid dehydrogenase 1 inhibitor, was administered once daily for 14 days to eight patients with type 2 diabetes mellitus. N-methylation was identified as a major biotransformation pathway. In four patients treated with BI 187004, the plasma exposure
Influence of 1-methylbenzimidazole interactions with Li+ and TiO 2 on the performance of dye-sensitized solar cells.
Zhang C, et al.
Electrochimica Acta, 53(17), 5503-5508 (2008)
Yanyan Fang et al.
Chemistry, an Asian journal, 14(23), 4201-4206 (2019-10-10)
Two types of ionic liquids (ILs), 1-(3-hexenyl)-3-methyl imidazolium iodide and 1-(3-butenyl)-3-methyl imidazolium iodide, are synthesized by introducing an unsaturated bond into the side alkyl chain of the imidazolium cation. These new ionic liquids exhibit high thermal stability and low viscosity
Kelvin K H Tong et al.
Molecules (Basel, Switzerland), 25(16) (2020-08-17)
Thiones have been investigated as ligands in metal complexes with catalytic and biological activity. We report the synthesis, characterization, and biological evaluation of a series of MII/III complexes of the general formulae [MII(cym)(L)Cl]X (cym = η6-p-cymene) or [MIII(Cp*)(L)Cl]X (Cp* =
Optimization of dye-sensitized solar cells prepared by compression method.
Boschloo G, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 148(1), 11-15 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service