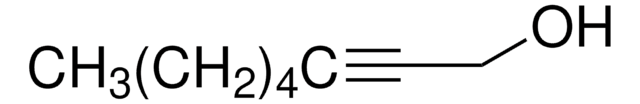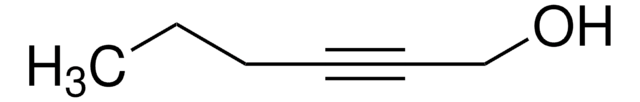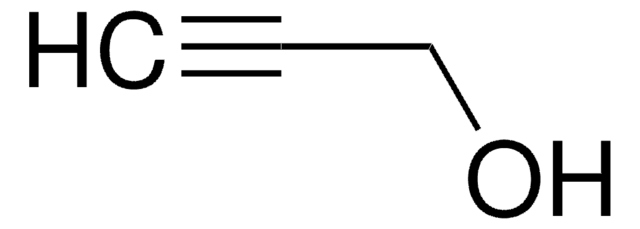127280
1-Octyn-3-ol
96%
Synonym(s):
(RS)-1-Octyn-3-ol, (±)-1-Octyn-3-ol, 1-Ethynyl-1-hexanol, 3-Hydroxyoct-1-yne
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.441 (lit.)
bp
83 °C/19 mmHg (lit.)
density
0.864 g/mL at 25 °C (lit.)
SMILES string
CCCCCC(O)C#C
InChI
1S/C8H14O/c1-3-5-6-7-8(9)4-2/h2,8-9H,3,5-7H2,1H3
InChI key
VUGRNZHKYVHZSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Octyn-3-ol is a racemic intermediate formed during the synthesis of enantiomerically pure secondary alcohols with sterically similar substituents.
Application
1-Octyn-3-ol was used in the synthesis of synthetic tricolorin A, a novel tetrasaccharide macrolactone that is a natural herbicide†.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
José-Luis Abad et al.
The Journal of organic chemistry, 68(13), 5351-5356 (2003-06-21)
A novel chemoenzymatic strategy for the synthesis of enantiomerically pure secondary alcohols with sterically similar substituents is described. The key step is the kinetic lipase-catalyzed resolution of racemic mixtures of substituted propargylic alcohols. The efficiency of this new approach was
Daniel P. Larson et al.
The Journal of organic chemistry, 62(24), 8406-8418 (2001-10-24)
Tricolorin A (1) is a novel tetrasaccharide macrolactone that is a natural herbicide. In this paper is reported a total synthesis of 1. Coupling of hydroxy ester 18 with D-fucosyl trichloroacetimidate 23 gave fucoside 24. Removal of the C-2 pivaloyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service