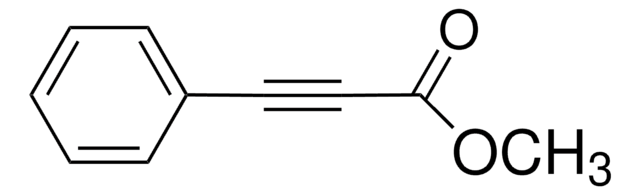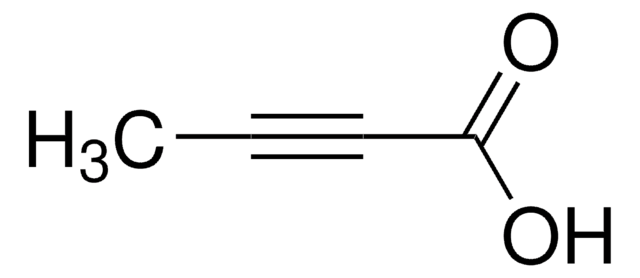171859
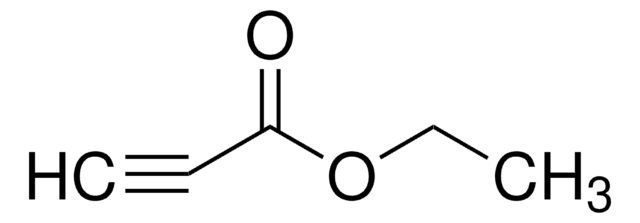
Methyl propiolate
99%
Synonym(s):
Methyl acetylenecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HC≡CCO2CH3
CAS Number:
Molecular Weight:
84.07
Beilstein:
605462
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.408 (lit.)
bp
103-105 °C (lit.)
density
0.945 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
COC(=O)C#C
InChI
1S/C4H4O2/c1-3-4(5)6-2/h1H,2H3
InChI key
IMAKHNTVDGLIRY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-3 protecting reagent for uridines and thymidines.
Methyl propiolate was used in the synthesis of polysubstituted 3-arylaminoacrylate and tetrahydropyrimidin-2-one derivatives. It was also used as a thiol derivatizing agent for capillary electrophoresis.
Used in a one-pot, four-component synthesis of 1,2,3,5-benzenetetracarboxylates promoted by Ph3P.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Eirini-Chrysanthi Tsardaka et al.
Journal of chromatography. A, 1300, 204-208 (2013-06-04)
In this study we demonstrate - for the first time - the suitability of methyl propiolate - an alkylester of propiolic acid - as a thiol derivatizing reagent for capillary electrophoresis. Glutathione (as analyte in yeast samples) and N-acetylcysteine (as
Tetrahedron Letters, 36, 3261-3261 (1995)
Li-Li Zhang et al.
Molecular diversity, 18(1), 79-89 (2014-01-31)
Polysubstituted 3-arylaminoacrylate and tetrahydropyrimidin-2-one derivatives could be selectively produced from the one-pot domino reaction of arylamines, methyl propiolate, aromatic aldehydes, and urea in ethanol in the presence of FeCl3 as catalyst. Under similar reactions secondary amines such as morpholine and
Graziella Tocco et al.
Journal of agricultural and food chemistry, 68(40), 11088-11095 (2020-09-15)
The present study reports on the powerful nematicidal activity of a series of electron-deficient alkynes against the root-knot nematode Meloidogyne incognita (Kofoid and White) Chitwood. Interestingly, we found that the conjugation of electron-withdrawing carbonyl groups to an alkyne triple bond
Helvetica Chimica Acta, 89, 2918-2918 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service