D9260
Dextran from Leuconostoc mesenteroides
average mol wt 9,000-11,000
About This Item
Recommended Products
biological source
bacterial (Leuconostoc mesenteroides)
Quality Level
form
powder
optical activity
[α]/D 199°
mol wt
average mol wt 9,000-11,000
solubility
water: 0.1 g/mL, colorless to faintly yellow
storage temp.
2-8°C
InChI
1S/C18H32O16/c19-1-5(21)9(23)10(24)6(22)3-31-17-16(30)14(28)12(26)8(34-17)4-32-18-15(29)13(27)11(25)7(2-20)33-18/h1,5-18,20-30H,2-4H2
InChI key
FZWBNHMXJMCXLU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Dextrans of less than 60,000Da are generally considered low molecular weight dextrans. Low molecular weight dextrans are often preferred versus high molecular weight dextrans due to their viscosities, aggregation and permeation properties. High molecular weight, water-soluble, dextran polymers have been used in a wide variety of biomedical applications. Dextran (9,000-11,000), a low molecular weight dextran, may be used to study processes such as transient plasma membrane and blood brain barrier (BBB) permeabilization. It may be used as a sugar based crowding agent in protein packing and folding studies.
Preparation Note
Other Notes
Storage Class Code
11 - Combustible Solids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Dextran sulfates are supplied as the sodium salt forms, making them soluble and stable in water. Dextran sulfate contains approximately 17% sulfur which is equivalent to approximately 2.3 sulfate groups per glucosyl residue.
Dextran sulfates are supplied as the sodium salt forms, making them soluble and stable in water. Dextran sulfate contains approximately 17% sulfur which is equivalent to approximately 2.3 sulfate groups per glucosyl residue.
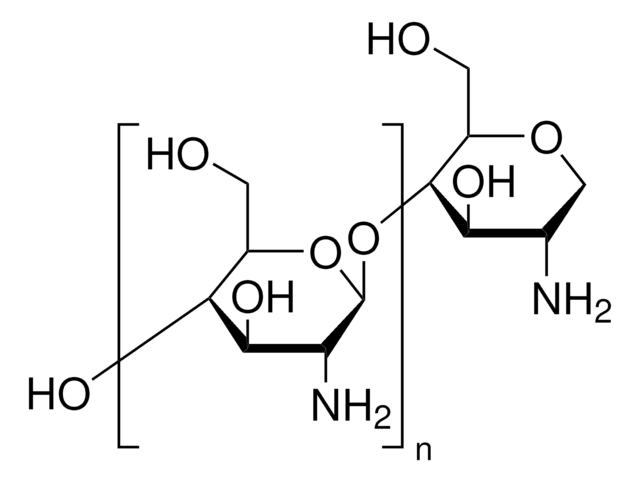
Dextran is a polymer of anhydroglucose. It is composed of approximately 95% alpha-D-(1-6) linkages. The remaining a(1-3) linkages account for the branching of dextran. Conflicting data on the branch lengths implies that the average branch length is less than three glucose units. However, other methods indicate branches of greater than 50 glucose units exist.
Dextran sulfates are supplied as the sodium salt forms, making them soluble and stable in water. Dextran sulfate contains approximately 17% sulfur which is equivalent to approximately 2.3 sulfate groups per glucosyl residue.
Related Content
Learn about C6H10O6 or dextran formation, classes and naming from MilliporeSigma. Dextrans are polysaccharides with molecular weights ≥1,000 Dalton, which have a linear backbone of α-linked d-glucopyranosyl repeating units.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service