About This Item
Recommended Products
form
solid
reaction suitability
reaction type: C-C Bond Formation
InChI
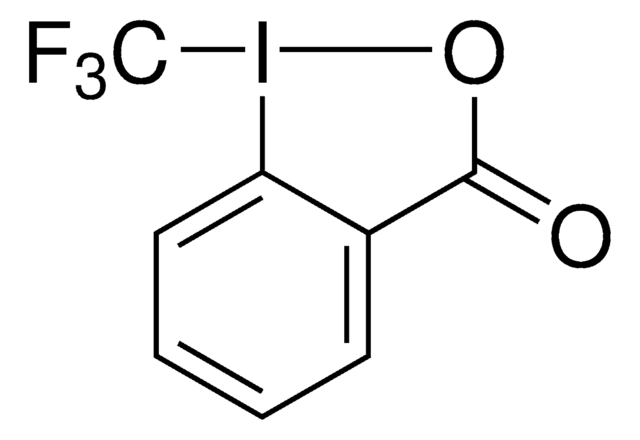
1S/C9H10ClIO/c1-9(2)7-5-3-4-6-8(7)11(10)12-9/h3-6H,1-2H3
InChI key
HEBYOYIIQNKPGQ-UHFFFAOYSA-N
Application
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service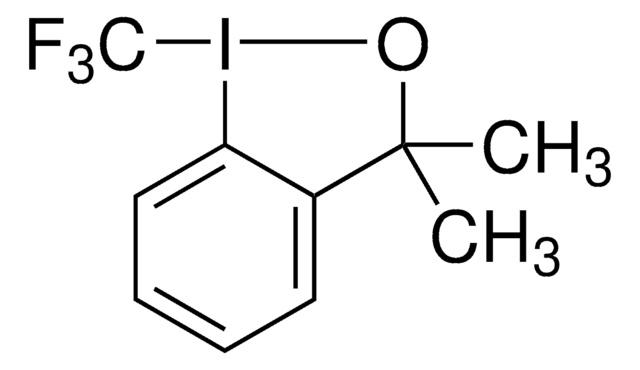
![1-((3,5-Difluorophenyl)sulfonyl)bicyclo[1.1.0]butane 95%](/deepweb/assets/sigmaaldrich/product/structures/640/054/e4a2fe87-4239-49ea-be2f-1273316c36ba/640/e4a2fe87-4239-49ea-be2f-1273316c36ba.png)
![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)

