152986
1-Benzyl-4-hydroxypiperidine
96%
Synonym(s):
1-Benzyl-4-piperidinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H17NO
CAS Number:
Molecular Weight:
191.27
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
bp
127-128 °C/2 mmHg (lit.)
mp
61-63 °C (lit.)
functional group
hydroxyl
phenyl
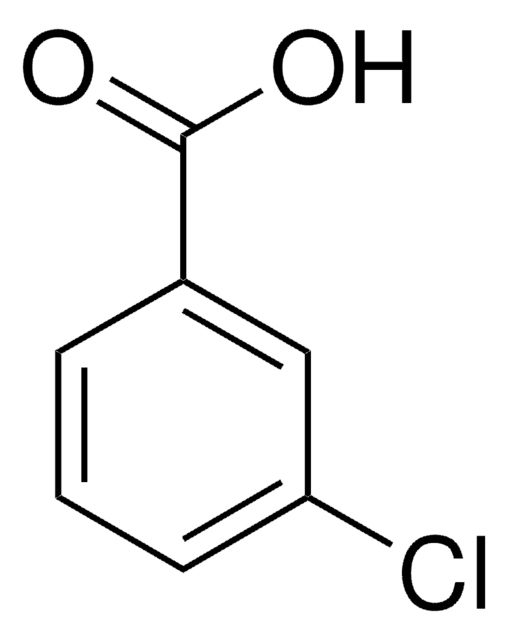
SMILES string
OC1CCN(CC1)Cc2ccccc2
InChI
1S/C12H17NO/c14-12-6-8-13(9-7-12)10-11-4-2-1-3-5-11/h1-5,12,14H,6-10H2
InChI key
BPPZXJZYCOETDA-UHFFFAOYSA-N
Application
1-Benzyl-4-hydroxypiperidine was used as an alternative molecule to study the ligand concentration attached to the epoxy-activated Sepharose 6B.
Reactant for synthesis of:
Muscarinic acetylcholine receptor antagonist and beta 2 adrenoceptor agonist
Fatty acid amide hydrolase inhibitors
PI3 kinase-alpha inhibitors
Flavonoid derivatives used as dual binding acetylcholinesterase inhibitors
Urotensin-II receptor antagonists
Rho kinase inhibitors
Muscarinic acetylcholine receptor antagonist and beta 2 adrenoceptor agonist
Fatty acid amide hydrolase inhibitors
PI3 kinase-alpha inhibitors
Flavonoid derivatives used as dual binding acetylcholinesterase inhibitors
Urotensin-II receptor antagonists
Rho kinase inhibitors
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
X Santarelli et al.
Journal of chromatography. B, Biomedical sciences and applications, 739(1), 63-72 (2000-04-01)
New pseudo-affinity chromatographic supports for penicillin acylase were prepared and evaluated with three different samples: pure penicillin acylase, industrial clarified feedstock and crude extract. The different gels were studied for their purification fold (three to six) and their recovery power
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service