A7811
Monoclonal Anti-α-Actinin (Sarcomeric) antibody produced in mouse
clone EA-53, ascites fluid
Synonym(s):
Actinin Antibody, Sarcomeric Alpha Actinin Antibody, Sarcomeric Alpha Actinin Antibody - Monoclonal Anti-α-Actinin (Sarcomeric) antibody produced in mouse, Anti-1110008F24Rik, Anti-ACTN2, Anti-Actinin α2, Anti-CMD1AA, Anti-MGC107582
About This Item
Recommended Products
biological source
mouse
Quality Level
conjugate
unconjugated
antibody form
ascites fluid
antibody product type
primary antibodies
clone
EA-53, monoclonal
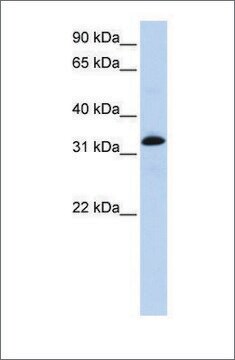
mol wt
antigen 100 kDa
contains
15 mM sodium azide
species reactivity
mouse, human, frog, pig, feline, chicken, hamster, canine, bovine, fish, snake, rabbit, sheep, goat, rat, lizard
technique(s)
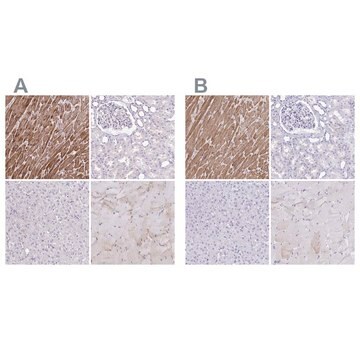
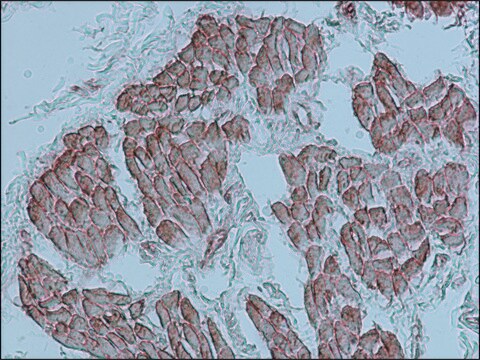
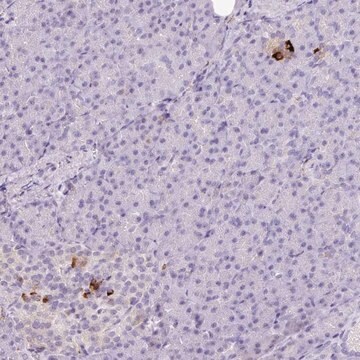
immunohistochemistry (formalin-fixed, paraffin-embedded sections): 1:800 using human skeletal and cardiac muscle
indirect ELISA: suitable using human and animal muscle tissue
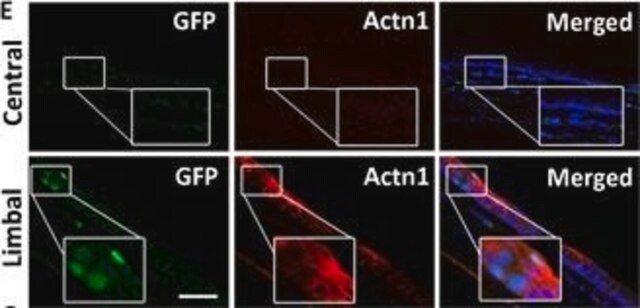
indirect immunofluorescence: suitable using human and animal cultured muscle cells
western blot: 1:2,500 using rat leg muscle
isotype
IgG1
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... ACTN2(88)
mouse ... Actn2(11472)
rat ... Actn2(291245)
General description
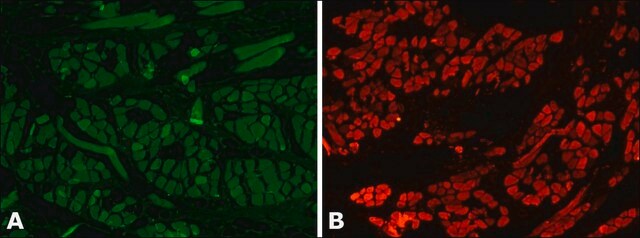
Mouse monoclonal anti-α-actinin (sarcomeric) antibody stains thymic myoid cells. The antibody exhibits wide reactivity with human and animal muscle tissue.
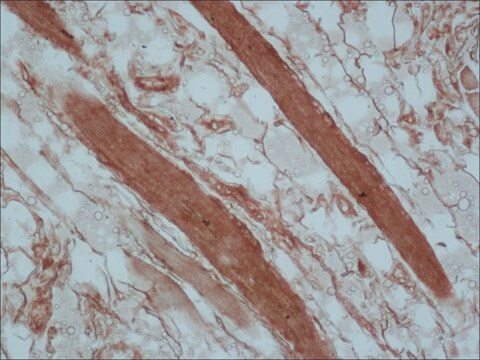
The antibody is specific for a-skeletal and a-cardiac muscle actinins. The antibody labels Z lines and dots in stress fibers of skeletal muscle in myotubes but does not react with non-skeletal muscle elements (e.g., connective tissue, epithelium, nerves, smooth muscle).
Mouse monoclonal anti-α-actinin (sarcomeric) antibody stains thymic myoid cells. The antibody exhibits wide reactivity with human and animal muscle tissue. α-actinin is detected predominantly in dense bodies and plaques which are characteristic of that tissue. Immunofluorescent labeling of a large variety of cells with anti a -actinin reveals an extensive association of the proteins with the actin containing stress fibers and, in particular, with their membrane-bound termini.
Monoclonal Anti-α-Actinin (Sarcomeric) (mouse IgG1 isotype) is derived from the EA-53 hybridoma produced by the fusion of mouse myeloma cells and splenocytes from BALB/c mice immunized with purified rabbit skeletal a-actinin.1 The isotype is determined using Mouse Monoclonal Antibody Isotyping Reagents, Catalog Number ISO2. Monoclonal Anti-α-Actinin (Sarcomeric) may be used for the localization of sarcomeric α-actinin using various immunochemical assays such as ELISA, dot blot, immunoblot, immunohistochemistry, and immunocytochemistry. The antibody is useful in the immunolocalization of α-actinin in normal and neoplastic cultured cells and tissues, and for studies on the state of sarcomeric muscle organization, in normal and pathological situations.
Specificity
Immunogen
Application
Biochem/physiol Actions
Physical form
Storage and Stability
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
related product
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
High titer lentiviral particles including beta-actin, alpha-tubulin and vimentin used for live cell analysis of cytoskeleton structure proteins.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service