推荐产品
等級
pharmaceutical primary standard
API 家族
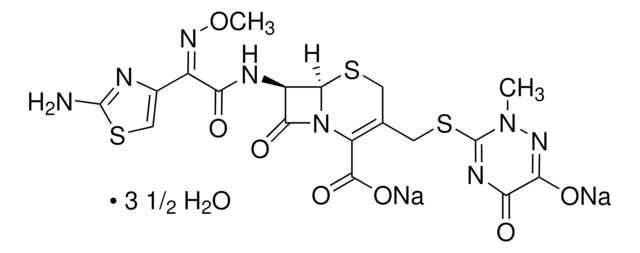
ceftriaxone
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
O.O.O.O.O.O.O.[Na+].[Na+].CO\N=C(/C(=O)N[C@H]1C2SCC(CSC3=NC(=O)C(=O)N([Na])N3C)=C(N2C1=O)C([O-])=O)c4csc(N)n4.CO\N=C(/C(=O)N[C@H]5C6SCC(CSC7=NC(=O)C(=O)N([Na])N7C)=C(N6C5=O)C([O-])=O)c8csc(N)n8
InChI
1S/2C18H18N8O7S3.4Na.7H2O/c2*1-25-18(22-12(28)13(29)23-25)36-4-6-3-34-15-9(14(30)26(15)10(6)16(31)32)21-11(27)8(24-33-2)7-5-35-17(19)20-7;;;;;;;;;;;/h2*5,9,15H,3-4H2,1-2H3,(H5,19,20,21,23,27,29,31,32);;;;;7*1H2/q;;4*+1;;;;;;;/p-4/b2*24-8-;;;;;;;;;;;/t2*9-,15-;;;;;;;;;;;/m11.........../s1
InChI 密鑰
PMRZKYOXTPBIQF-MAODNAKNSA-J
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- Ceftriaxone for Injection
- Ceftriaxone Injection
分析報告
其他說明
相關產品
訊號詞
Danger
危險分類
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门