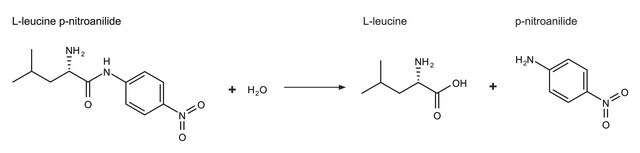
推荐产品
重組細胞
expressed in E. coli
品質等級
化驗
≥93% (SDS-PAGE)
形狀
solution
比活性
0.5 units/mg protein
分子量
37 kDa by SDS-PAGE
UniProt登錄號
異物活動
Other proteases, none detected
運輸包裝
dry ice
儲存溫度
−20°C
基因資訊
Pyrococcus furiosus DSM 3638 ... PF0541(1468383)
相关类别
一般說明
Methionine aminopeptidase from Pyrococcus furiosus is a 32 kDa thermostable enzyme. It belongs to type 2a class of methionine aminopeptidase. Methionine aminopeptidase maintains protein homeostasis and coordinates posttranslational modification of proteins in eukaryotes.
X-ray crystallography of the structure of methionine aminopeptidase from Pyrococcus furiosus or PfMAP was performed at a resolution of 1.75A and showed that the protein consists of a catalytic domain containing two cobalt ions in the active site and a unique insertion domain which is specific to the prokaryotic form of the protein.
應用
Methionine Aminopeptidase from Pyrococcus furiosus has been used in a study to analyze the binding of Co(II)-specific inhibitors to the methionyl aminopeptidases from Escherichia coli and Pyrococcus furiosus. It has also been used in a study to examine the binding of a new class of pseudopeptide analog inhibitors.
生化/生理作用
Thermostable methionine aminopeptidase, which specifically liberates the N-terminal methioinine from proteins and peptides.
單位定義
One unit will hydrolyze 1 μmol of Met from Met-Pro-Ala-Ala-Gly in 1 minute at pH 7.5 at 37 °C.
外觀
Solution containing 0.01% Tween® 20, 0.1 mM CoCl2, and 10 mM Tris-HCl, pH 7.5.
法律資訊
TWEEN is a registered trademark of Croda International PLC
儲存類別代碼
12 - Non Combustible Liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Methionine aminopeptidase from the hyperthermophilic Archaeon Pyrococcus furiosus: molecular cloning and overexperssion in Escherichia coli of the gene, and characteristics of the enzyme
Tsunasawa S, et al.
Journal of Biochemistry, 122(4), 843-850 (1997)
Advances in bacterial methionine aminopeptidase inhibition
Helgren TR, et al.
Current Topics in Medicinal Chemistry, 16(4), 397-414 (2016)
S Tsunasawa et al.
Journal of biochemistry, 122(4), 843-850 (1997-12-17)
A gene for a methionine aminopeptidase (MAP; EC 3.4.11.18), which catalyzes the removal of amino-terminal methionine from the growing peptide chain on the ribosome, has been cloned from the hyperthermophilic Archaeon, Pyrococcus furiosus, by a novel method effectively using its
T H Tahirov et al.
Journal of molecular biology, 284(1), 101-124 (1998-11-13)
The structure of methionine aminopeptidase from hyperthermophile Pyrococcus furiosus (PfMAP) with an optimal growth temperature of 100 degreesC was determined by the multiple isomorphous replacement method and refined in three different crystal forms, one monoclinic and two hexagonal, at resolutions
Sanghamitra Mitra et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 14(4), 573-585 (2009-02-10)
Methionine aminopeptidases (MetAPs) represent a unique class of protease that is capable of the hydrolytic removal of an N-terminal methionine residue from nascent polypeptide chains. MetAPs are physiologically important enzymes; hence, there is considerable interest in developing inhibitors that can
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门