推荐产品
化驗
≥98% (HPLC)
形狀
powder
技術
HPLC: suitable
顏色
white to off-white
溶解度
water: 5 mg/mL, clear, colorless
儲存溫度
−20°C
SMILES 字串
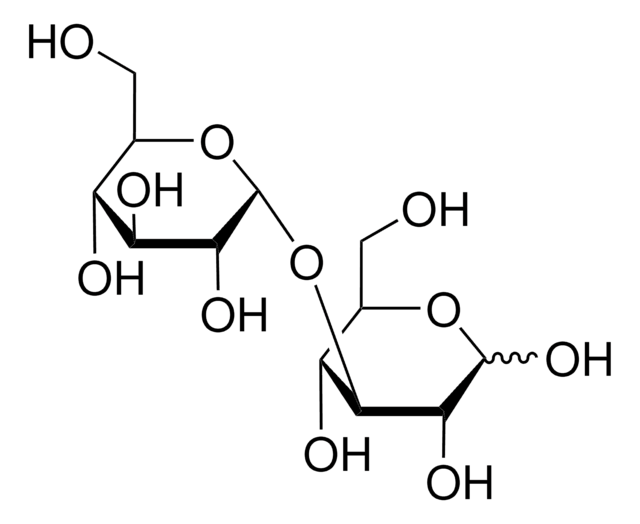
OCC(O)C(O)C(O)C(OC1OC(CO)C(O)C(O)C1O)C=O
InChI
1S/C12H22O11/c13-1-4(16)7(17)8(18)5(2-14)22-12-11(21)10(20)9(19)6(3-15)23-12/h2,4-13,15-21H,1,3H2
InChI 密鑰
PZDOWFGHCNHPQD-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
應用
Kojibiose, a disaccharide product of glucose caramelization and an inhibitor of plant glucosidase I, may be used to help identify and characterize glucosidase I enzymes involved in terminal deglycosylation of high-mannose oligosaccharides. Kojibiose may be used as a substrate to study the biological species, enzymes and catabolic processes that catabolize it as an energy source. Kojibiose may be used to identify, differentiate and characterize kojibiose phosphorylase(s) (KP).
生化/生理作用
Kojibiose is an inhibitor of plant glucosidase I. It inhibits the removal of terminal glucose from (Glc)3(Man)9(GlcNAc)2.
Kojibiose is an inhibitor of plant glucosidase I. It inhibits the removal of terminal glucose from the high-mannose oligosaccharide (Glc)3(Man)9(GlcNAc)2, either from the free oligosaccharide or from the oligosaccharide attached to a protein via N-linkage.
其他說明
To gain a comprehensive understanding of our extensive range of Disaccharides for your research, we encourage you to visit our Carbohydrates Category page.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Jong-Hyun Jung et al.
Journal of bacteriology, 196(5), 1122-1131 (2014-01-07)
A unique gene cluster responsible for kojibiose utilization was identified in the genome of Pyrococcus sp. strain ST04. The proteins it encodes hydrolyze kojibiose, a disaccharide product of glucose caramelization, and form glucose-6-phosphate (G6P) in two steps. Heterologous expression of
Satoshi Okada et al.
The FEBS journal, 281(3), 778-786 (2013-11-22)
Glycoside hydrolase (GH) family 65 contains phosphorylases acting on maltose (Glc-α1,4-Glc), kojibiose (Glc-α1,2-Glc), trehalose (Glc-α1,α1,-Glc), and nigerose (Glc-α1,3-Glc). These phosphorylases can efficiently catalyze the reverse reactions with high specificities, and thus can be applied to the practical synthesis of α-glucosyl
M K Dowd et al.
Carbohydrate research, 230(2), 223-244 (1992-06-16)
Energy surfaces were computed for relative orientations of the relaxed pyranosyl rings of the two anomeric forms of kojibiose, nigerose, and maltose, the (1----2)-alpha, (1----3)-alpha, and (1----4)-alpha-linked D-glucosyl disaccharides, respectively. Twenty-four combinations of starting conformations of the rotatable side-groups were
Wouter F J Hogendorf et al.
Bioorganic & medicinal chemistry, 18(11), 3668-3678 (2010-04-23)
In this paper the synthesis of an Enterococcus Faecalis teichoic acid (TA) hexamer is presented. The key kojibiosyl-glycerol phosphoramidite building block was obtained by condensation of thioglucose donors, provided with various protecting groups at the C2 hydroxyl function with an
A R Santa Cruz et al.
Experientia, 41(7), 928-929 (1985-07-15)
We have prepared dolichylpyrophosphoryl-[14C]-oligosaccharide (Dol-PP-oligosaccharide) from calf thyroid. Microsomal fractions from human breast tissues catalyzed the transfer of labeled oligosaccharide to endogenous acceptor proteins. Malignant tumors showed higher activity of the oligosaccharide transferring enzyme than normal tissue. With kojibiose (Kj)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门