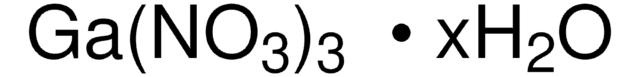G5169
GGTI 298 trifluoroacetate salt hydrate
≥90% (HPLC), film
别名:
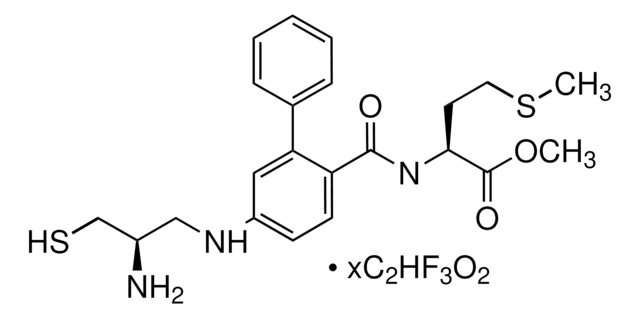
N-[[4-(2-(R)-Amino-3-mercaptopropyl)amino]-2-naphthylbenzoyl]leucine methyl ester trifluoroacetate salt hydrate
About This Item
推荐产品
品質等級
化驗
≥90% (HPLC)
形狀
film
lyophilized
雜質
<10% dimer
顏色
colorless
mp
99.5-100 °C
溶解度
DMSO: >20 mg/mL
儲存溫度
−20°C
SMILES 字串
OC(=O)C(F)(F)F.COC(=O)[C@H](CC(C)C)NC(=O)c1ccc(NC[C@@H](N)CS)cc1-c2cccc3ccccc23
InChI
1S/C27H33N3O3S.C2HF3O2/c1-17(2)13-25(27(32)33-3)30-26(31)23-12-11-20(29-15-19(28)16-34)14-24(23)22-10-6-8-18-7-4-5-9-21(18)22;3-2(4,5)1(6)7/h4-12,14,17,19,25,29,34H,13,15-16,28H2,1-3H3,(H,30,31);(H,6,7)/t19-,25+;/m1./s1
InChI 密鑰
WALKWJPZELDSKT-UFABNHQSSA-N
基因資訊
human ... PGGT1B(5229)
應用
- to study the anticancer effects of statins along with GGTI 298
- to study its combinatorial effects with FTI-277 on statin-mediated activation of extracellular signal-regulated kinase 5 (ERK5) in the human endothelium
- to evaluate the effect of protein geranylgeranylation (GG) inhibition on the breast cancer stem cell (CSC) population
生化/生理作用
特點和優勢
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门