所有图片(1)
About This Item
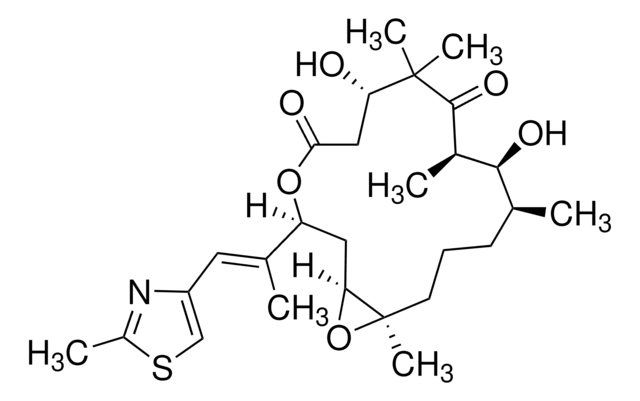
经验公式(希尔记法):
C26H39NO6S
CAS号:
分子量:
493.66
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推荐产品
生物源
Sorangium cellulosum
化驗
>95% (HPLC)
形狀
solid
儲存條件
desiccated
protect from light
儲存溫度
−20°C
SMILES 字串
O=C(O[C@@](/C(C)=C/C1=CSC(C)=N1)([H])C[C@@](O2)([H])[C@@]2([H])CCC[C@H](C)[C@H](O)[C@H]3C)C[C@H](O)C(C)(C)C3=O
InChI
1S/C26H39NO6S/c1-14-8-7-9-19-21(32-19)11-20(15(2)10-18-13-34-17(4)27-18)33-23(29)12-22(28)26(5,6)25(31)16(3)24(14)30/h10,13-14,16,19-22,24,28,30H,7-9,11-12H2,1-6H3/b15-10+/t14-,16+,19+,20-,21-,22-,24-/m0/s1
InChI 密鑰
HESCAJZNRMSMJG-KKQRBIROSA-N
生化/生理作用
(-)-Epothilone A is a microtubule (MT) stabilizing drug and natural macrolide antitumor from myxobacteria Sorangium cellulosum. EpoA exhibits kinetics similar to paclitaxel by inducing tubulin polymerization in vitro and producing enhanced microtubule stability and bundling in cultured cells. In contrast to paclitaxel, Epothilone A exhibits a greater cytotoxicity against P-glycoprotein-expressing multidrug resistant (MDR) cells (IC50 = 20 nM for MDR CCRF-CEM/VBL100 cells). Epothilone A is a competitve inhibitor of 3H-paclitaxel binding with comparable IC50 to paclitaxel in displacement competition assays. EpoA causes cell cycle arrest at the G2/M transition leading to cytotoxicity.
EpoA is a microtubule stabilizing drug and natural macrolide antitumor.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Daniele Passarella et al.
Bioorganic & medicinal chemistry, 17(21), 7435-7440 (2009-10-07)
The preparation and biological evaluation of a novel series of dimeric epothilone A derivatives (1-6) are described. Two types of diacyl spacers were introduced to establish the various dimeric epothilone A constructs. The effect of these compounds on tubulin polymerization
Bernhard Pfeiffer et al.
Bioorganic & medicinal chemistry letters, 19(14), 3760-3763 (2009-05-13)
The SAR of a series of new epothilone A derivatives with a 2-substituted-1,3-oxazoline moiety trans-fused to the C12-C13 bond of the deoxy macrocycle have been investigated with regard to tubulin polymerization induction and cancer cell growth inhibition. Significant differences in
Isao Kobayashi et al.
Scientific reports, 9(1), 14205-14205 (2019-10-04)
Hematopoietic stem cells (HSCs) maintain the entire blood system throughout life and are utilized in therapeutic approaches for blood diseases. Prospective isolation of highly purified HSCs is crucial to understand the molecular mechanisms underlying regulation of HSCs. The zebrafish is
Joys of molecules. 2. Endeavors in chemical biology and medicinal chemistry.
K C Nicolaou
Journal of medicinal chemistry, 48(18), 5613-5638 (2005-09-02)
Máté Erdélyi et al.
Journal of medicinal chemistry, 51(5), 1469-1473 (2008-02-15)
The conformational properties of the microtubule-stabilizing agent epothilone A ( 1a) and its 3-deoxy and 3-deoxy-2,3-didehydro derivatives 2 and 3 have been investigated in aqueous solution by a combination of NMR spectroscopic methods, Monte Carlo conformational searches, and NAMFIS calculations.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门