推荐产品
形狀
powder
品質等級
儲存溫度
2-8°C
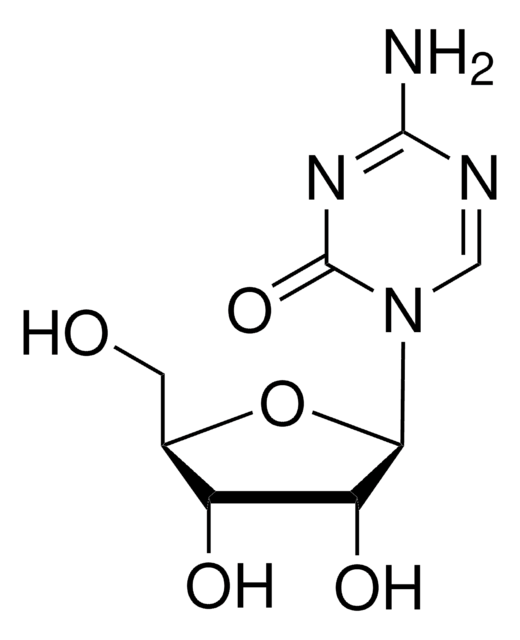
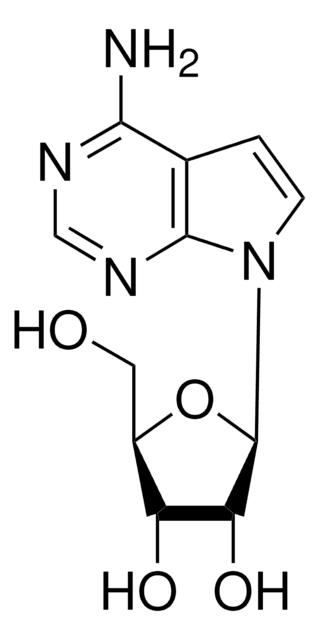
SMILES 字串
Nc1nccc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C11H14N4O4/c12-10-7-5(1-2-13-10)15(4-14-7)11-9(18)8(17)6(3-16)19-11/h1-2,4,6,8-9,11,16-18H,3H2,(H2,12,13)/t6-,8-,9-,11-/m1/s1
InChI 密鑰
DBZQFUNLCALWDY-PNHWDRBUSA-N
基因資訊
rat ... Adora1(29290) , Adora2a(25369) , Adora3(25370)
應用
3-Deazaadenosine has been used as a methylation inhibitor:
- to study the effect of m6A modification on suppressor of cytokine signaling 2 (SOCS2) expression in colon cancer cells
- to study its effects on the expression of influenza A virus (IAV) proteins in human lung epithelial cell line
- to evaluate its effects on the replication of SV40 virus in BSC40 cells
生化/生理作用
3-Deazaadenosine (DZA), an analog of adenosine, acts as a blocker of S-adenosylhomocysteine (SAH)-hydrolase, a regulator of cellular methyltransferase activity. It has been observed in inhibiting some of the factors involved in atherosclerosis and restenosis. DZA inhibits lymphocyte-mediated tumor cell lysis, macrophage phagocytosis, microfilament disorganization, monocyte, and neutrophil chemotaxis. It also inhibits histamine release by basophils, superoxide anion generation, and macrophage lysosomal secretion. DZA possesses anti-inflammatory, anti-human immunodeficiency virus (HIV) properties and inhibitory effects of cytokine expression that include interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and nuclear factor-κB (NF-κB) transcription activity.
Possesses antiviral activity inhibitor of leukocyte adhesion to TNF-treated endothelial cells.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Janusz M Bujnicki et al.
Proteins, 52(4), 624-632 (2003-08-12)
S-adenosylhomocysteine hydrolase (SAHH) is a key regulator of S-adenosylmethionine-dependent methylation reactions and an interesting pharmacologic target. We cloned the SAHH gene from Plasmodium falciparum (PfSAHH), with an amino acid sequence agreeing with that of the PlasmoDB genomic database. Even though
Daniel G Sedding et al.
Circulation research, 104(10), 1192-1200 (2009-04-18)
3-Deazaadenosine (c3Ado) is a potent inhibitor of S-adenosylhomocysteine hydrolase, which regulates cellular methyltransferase activity. In the present study, we sought to determine the effect of c3Ado on vascular smooth muscle cell (VSMC) function and neointima formation in vivo. c3Ado dose-dependently
Ruediger C Braun-Dullaeus et al.
Shock (Augusta, Ga.), 19(3), 245-251 (2003-03-13)
Severe sepsis is accompanied by a profound depression of myocardial contractility. Leukocyte adhesion with subsequent local excess nitric oxide and reactive oxygen species production play major roles for this deleterious effect. We hypothesized that 3-deazaadenosine (c3Ado), an adenosine analogue with
Diane M Hill et al.
Clinical chemistry, 48(11), 2017-2022 (2002-10-31)
The accuracy of homocysteine (Hcy) results is currently compromised by the requirement to separate the plasma within 1 h of sample collection. We studied the effect of temperature on the stability of plasma Hcy over a 72-h time course in
Lydia A Afman et al.
Brain research. Developmental brain research, 158(1-2), 59-65 (2005-07-06)
Periconceptional folic acid supplementation can reduce the occurrence of neural tube defects. A low folate status will result in reduced remethylation of homocysteine (Hcy) to methionine and, subsequently, in a rise of Hcy levels. Indeed, elevated Hcy concentrations have been
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门