推荐产品
形狀
crystalline powder
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
SMILES 字串
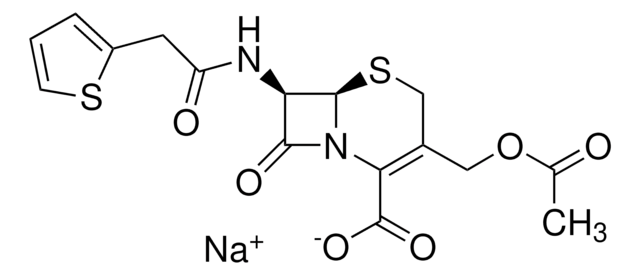
[Na+].CC(=O)OCC1=C(N2[C@H](SC1)[C@H](NC(=O)Cc3cccs3)C2=O)C([O-])=O
InChI
1S/C16H16N2O6S2.Na/c1-8(19)24-6-9-7-26-15-12(14(21)18(15)13(9)16(22)23)17-11(20)5-10-3-2-4-25-10;/h2-4,12,15H,5-7H2,1H3,(H,17,20)(H,22,23);/q;+1/p-1/t12-,15-;/m1./s1
InChI 密鑰
VUFGUVLLDPOSBC-XRZFDKQNSA-M
正在寻找类似产品? 访问 产品对比指南
一般說明
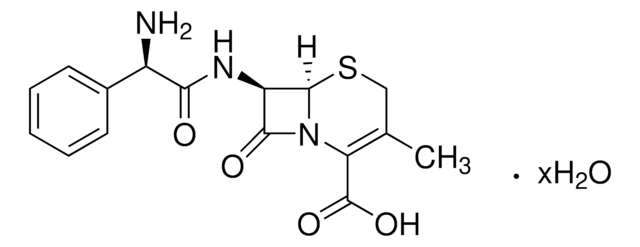
Chemical structure: ß-lactam
應用
Cephalothin is a first-generation cephalosporin antibiotic used to study the mechanism of liposome encapsulated antibiotics, strategies for co-opting β-lactamases of Gram-negative bacteria for treatment of antibiotics, and for immunology studies in relation to antibiotics. It is used to study the effect of expression, binding and inhibition of penicillin-binding proteins especially PBP6 on bacterial cell wall mucopeptide synthesis.
生化/生理作用
Mode of Action: Disrupts the synthesis of the peptidoglycan layer of bacterial cell walls.
Antimicrobial spectrum: Effective aginast both Gram-positive and Gram-negative bacteria.
Mode of Resistance: Production of cephalosporinase will inactivate the product.
Antimicrobial spectrum: Effective aginast both Gram-positive and Gram-negative bacteria.
Mode of Resistance: Production of cephalosporinase will inactivate the product.
其他說明
Keep container tightly closed in a dry and well-ventilated place. Keep in a dry place.
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
S Mobashery et al.
The Journal of biological chemistry, 261(17), 7879-7887 (1986-06-15)
Two novel C10-(dipeptidyl)cephalosporin esters (3-(beta-chloro-L-alanyl-beta-chloro-L-alanyloxymethyl)-7 beta-(2-thienylacetamido)-3-cephem-4-carboxylic acid (7) and sodium 3-(L-alanyl-L-alanyloxymethyl)-7 beta-(2-thienylacetamido)-3-cephem-4-carboxylate, toluene-sulfonic acid salt (18] were synthesized, and their reactions with Escherichia coli TEM beta-lactamase were examined. Kinetic parameters determined for the enzymatic reactions of 7 (Km = 0.32
Studies on the immune response to penicillin and cephalothin in humans. I. Optimal conditions for titration of hemagglutinating penicillin and cephalothin antibodies.
P Spath et al.
Journal of immunology (Baltimore, Md. : 1950), 107(3), 854-859 (1971-09-01)
J V Desiderio et al.
The Journal of infectious diseases, 148(3), 563-570 (1983-09-01)
Multilamellar liposomes (lipid bilayer vesicles) composed of phosphatidylcholine, cholesterol, and phosphatidylserine (molar ratio, 6:3:1) were produced and then made to entrap an aqueous solution of cephalothin. Resident murine peritoneal macrophages were shown to be capable of interiorizing the liposome-antibiotic complex;
Bulent Tokgoz et al.
Renal failure, 32(2), 179-184 (2010-03-05)
Aminoglycosides have been used in the treatment of CAPD peritonitis despite their potential risk for ototoxicity. The ototoxicity risk of intraperitoneally administered aminoglycosides has been investigated by a number of studies. However, their results are somewhat conflicting. The aim of
Smriti Sharma et al.
Journal of molecular modeling, 18(2), 481-492 (2011-05-05)
β-Lactamases are bacterial enzymes that act as a bacterial defense system against β-lactam antibiotics. β-Lactamase cleaves the β-lactam ring of the antibiotic by a two step mechanism involving acylation and deacylation steps. Although class C β-lactamases have been investigated extensively
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门