推荐产品
等級
pharmaceutical primary standard
API 家族
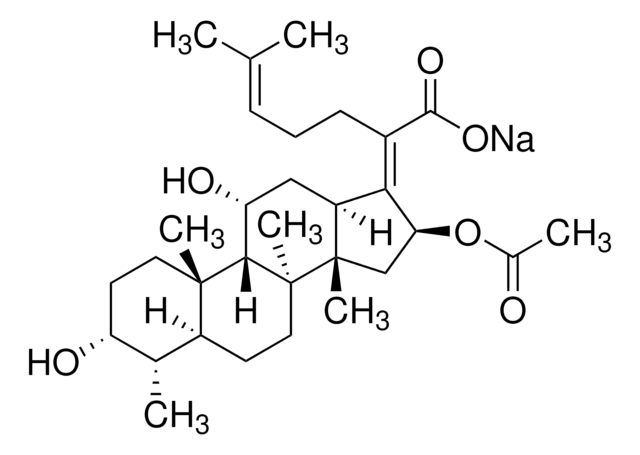
sodium fusidate
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
[Na+].C[C@@H]1[C@H](O)CC[C@@]2(C)C1CC[C@@]3(C)C2[C@H](O)CC4\C([C@H](C[C@]34C)OC(C)=O)=C(/CC\C=C(\C)C)C([O-])=O
InChI
1S/C31H48O6.Na/c1-17(2)9-8-10-20(28(35)36)26-22-15-24(34)27-29(5)13-12-23(33)18(3)21(29)11-14-30(27,6)31(22,7)16-25(26)37-19(4)32;/h9,18,21-25,27,33-34H,8,10-16H2,1-7H3,(H,35,36);/q;+1/p-1/b26-20-;/t18-,21-,22-,23+,24+,25-,27-,29-,30-,31-;/m0./s1
InChI 密鑰
HJHVQCXHVMGZNC-JCJNLNMISA-M
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Sodium fusidate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Suppresses nitric oxide lysis of pancreatic islet cells. Inhibits protein synthesis in prokaryotes by inhibiting the ribosome-dependent activity of G factor and translocation of peptidyl-tRNA.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Topical antibiotics in the treatment of superficial skin infections in general practice?a comparison of mupirocin with sodium fusidate.
White D G, et al.
The Journal of Infection, 18(3), 221-229 (1989)
Determination of fusidic acid and sodium fusidate in pharmaceutical dosage forms by first-derivative ultraviolet spectrophotometry.
Hassan S M, et al.
Analyst, 112(10), 1459-1461 (1987)
Saleem Shaikh et al.
Journal of chromatographic science, 47(2), 178-183 (2009-02-19)
A simple, specific, and precise high-performance liquid chromatographic method is developed and validated for the simultaneous determination of chlorocresol (CC), mometasone furoate (MF), and fusidic acid (FA) in a cream formulation. The isocratic mobile phase consists of 1.5% w/v aqueous
Katharina Wittmann et al.
Tissue engineering. Part A, 21(7-8), 1343-1353 (2015-01-21)
The development of vascularized and functional adipose tissue substitutes is required to improve soft tissue augmentation. In this study, vascularized adipose tissue constructs were generated using uncultured cells from the stromal-vascular fraction (SVF) of adipose tissue as an alternative cell
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门