推荐产品
化驗
≥95%
形狀
solid
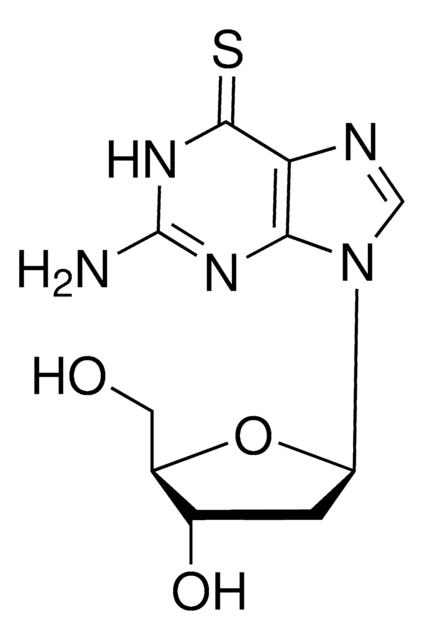
SMILES 字串
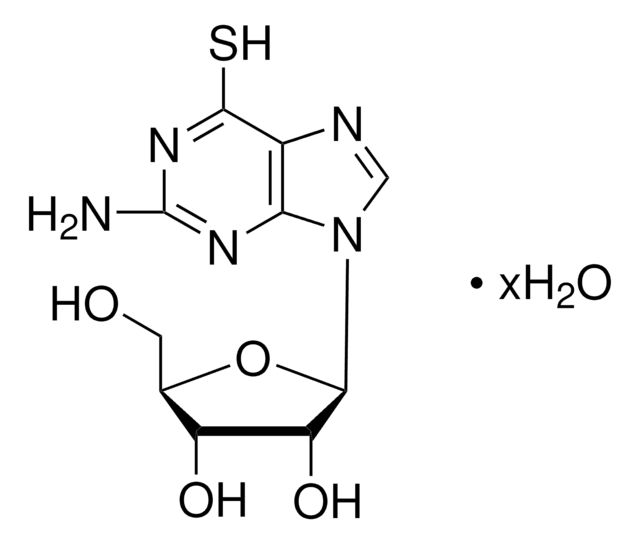
[H]n1cnc2c(SC)nc(N)nc12
InChI
1S/C6H7N5S/c1-12-5-3-4(9-2-8-3)10-6(7)11-5/h2H,1H3,(H3,7,8,9,10,11)
InChI 密鑰
YEGKYFQLKYGHAR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
2-氨基-6-甲基巯基嘌呤是一种2-氨基-6-烷基二硫代嘌呤,已与其他6位碳类似物一起用于研究大脑特异性地西泮的结合。
2-氨基-6-甲基巯基嘌呤(6-MTG)已用作Dulbecco′s改良的Eagles培养基(DMEM)培养基的补充剂,用于选择表达GPT的重组病毒mCMVhMIEPE-gpt.lacZ(巨细胞病毒主要立即早期启动子-增强子复合物-gpt.lacz)。它也已用作高效液相色谱(HPLC)中的标准品,以评估巯嘌呤甲基转移酶(TPMT)酶的活性。
生化/生理作用
2-氨基-6-甲基巯基嘌呤是由6-巯基嘌呤通过硫嘌呤甲基转移酶(TMPT)酶的S甲基化活性合成的。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
A Practical Non-Extraction Direct Liquid Chromatography Method for Determination of Thiopurine S-Methyltransferase Activity in Inflammatory Bowel Disease
Bahrehmand F, et al.
Acta Medica Iranica, 55(6), 360-367 (2017)
Effect of 9-alkyl derivatives of 6-methylthioguanine on brain specific binding of [3H]diazepam.
S C Sung et al.
Biochemical pharmacology, 35(20), 3645-3646 (1986-10-15)
S C Sung et al.
Biochemical pharmacology, 33(11), 1737-1739 (1984-06-01)
Various derivatives of 2-amino-6- methylthiopurine with substituents at the 6-position of purine were tested for their abilities to displace [3H]diazepam binding to rat brain membranes. The potency was dependent on the carbon chain-length in the 6-position of purine. Among the
P F Swann et al.
Science (New York, N.Y.), 273(5278), 1109-1111 (1996-08-23)
It is proposed here that the delayed cytotoxicity of thioguanine involves the postreplicative DNA mismatch repair system. After incorporation into DNA, the thioguanine is chemically methylated by S-adenosylmethionine to form S6-methylthioguanine. During DNA replication, the S6-methylthioguanine directs incorporation of either
S C Sung et al.
European journal of pharmacology, 81(3), 505-508 (1982-07-16)
We have compared fifteen synthetic purines and purine nucleosides on their ability to displace [3H]diazepam binding to rat brain membranes. Among these analogs, 6-methylthioguanine was found to be most potent, inhibiting competitively the specific binding of [3H]diazepam with a Ki
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门