推荐产品
蒸汽密度
3.5 (vs air)
品質等級
蒸汽壓力
6 mmHg ( 20 °C)
產品線
ReagentPlus®
化驗
≥99%
形狀
liquid
自燃溫度
662 °F
expl. lim.
11.4 %
折射率
n20/D 1.452 (lit.)
pH值
6 (20 °C, 200 g/L)
bp
140.4 °C (lit.)
mp
−23 °C (lit.)
密度
0.975 g/mL at 25 °C (lit.)
SMILES 字串
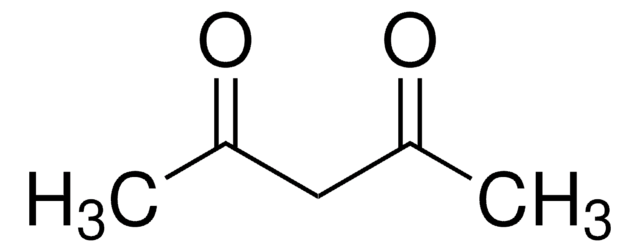
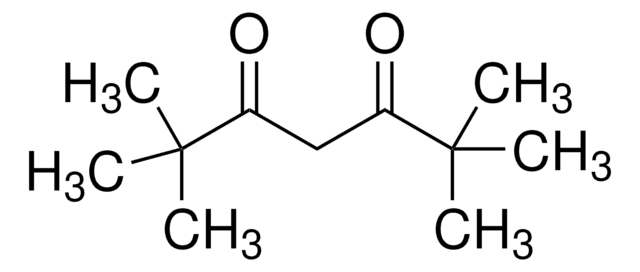
CC(=O)CC(C)=O
InChI
1S/C5H8O2/c1-4(6)3-5(2)7/h3H2,1-2H3
InChI 密鑰
YRKCREAYFQTBPV-UHFFFAOYSA-N
基因資訊
human ... ACHE(43) , BCHE(590) , CES1(1066)
正在寻找类似产品? 访问 产品对比指南
一般說明
乙酰丙酮(2,4-戊二酮)是一种含有两个羰基和一个活性亚甲基的有机化合物。它主要用作合成各种化学衍生物的中间体。它还可用作聚烯烃的改性剂、缓蚀剂和放射性示踪剂标记。
應用
乙酰丙酮可用作:
- 多功能配体,用于金纳米颗粒(AuNP)的合成和可行功能化。
- 反应物,通过与乙酰乙酸甲酯和 Morita-Baylis-Hillman 醋酸盐反应合成 9,10-二氢吖啶。
- 试剂,通过 Zr(OC3H7n)4 水解合成 ZrO2(二氧化锆)。乙酰丙酮可控制烷氧化物的水解和缩合速率,从而控制氧化物的成核和生长速率。
包裝
玻璃瓶封装
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
95.0 °F - closed cup
閃點(°C)
35 °C - closed cup
其他客户在看
Kyoji Tsuchikama et al.
The Journal of organic chemistry, 76(17), 6981-6989 (2011-06-18)
Bacteria have developed a cell-to-cell communication system, termed quorum sensing (QS), which allows for the population-dependent coordination of their behavior via the exchange of chemical signals. Autoinducer-2 (AI-2), a class of QS signals derived from 4,5-dihydroxy-2,3-pentandione (DPD), has been revealed
Heaweon Park et al.
Inorganic chemistry, 50(23), 11978-11989 (2011-11-01)
A series of high-spin iron(II) β-diketonato complexes have been prepared and characterized with the intent of modeling the substrate-bound form of the enzyme acetylacetone dioxygenase (Dke1). The Dke1 active site features an Fe(II) center coordinated by three histidine residues in
Rolando R Lozada-García et al.
Physical chemistry chemical physics : PCCP, 14(10), 3450-3459 (2012-02-07)
The photochemistry of the chelated enol form of acetylacetone (AcAc) was investigated by UV excitation of the S(2) state at 266 nm in parahydrogen matrices, complemented by experiments in neon and normal hydrogen matrices. Infrared (IR) spectroscopy, combined with theoretical
Zhenkun Sun et al.
Journal of the American Chemical Society, 134(42), 17653-17660 (2012-10-02)
The organization of different nano objects with tunable sizes, morphologies, and functions into integrated nanostructures is critical to the development of novel nanosystems that display high performances in sensing, catalysis, and so on. Herein, using acetylacetone as a chelating agent
Ivelina Georgieva et al.
Journal of molecular modeling, 18(6), 2409-2422 (2011-10-13)
Theoretical and spectroscopic studies of a series of monomeric and dimeric complexes formed through the modification of a zirconium butoxide precursor with acetylacetone and subsequent hydrolysis and/or condensation have been performed by applying DFT/B3LYP/6-31++G(d) and highly accurate RI-ADC(2) methods as
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门