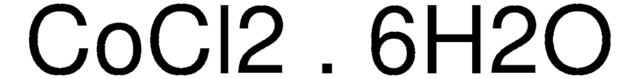所有图片(3)
About This Item
线性分子式:
CuCl2 · 2H2O
CAS号:
分子量:
170.48
EC號碼:
MDL號碼:
分類程式碼代碼:
12352302
PubChem物質ID:
NACRES:
NB.24
推荐产品
等級
ACS reagent
蒸汽密度
>1 (vs air)
化驗
≥99.0%
形狀
crystalline powder
crystals or granules
反應適用性
reagent type: catalyst
core: copper
雜質
≤0.01% insolubles
mp
100 °C (dec.) (lit.)
負離子痕跡
nitrate (NO3-): ≤0.015%
sulfate (SO42-): ≤0.005%
正離子痕跡
Ca: ≤0.005%
Fe: ≤0.005%
K: ≤0.01%
Na: ≤0.02%
Ni: ≤0.01%
SMILES 字串
Cl[Cu]Cl.[H]O[H].[H]O[H]
InChI
1S/2ClH.Cu.2H2O/h2*1H;;2*1H2/q;;+2;;/p-2
InChI 密鑰
MPTQRFCYZCXJFQ-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
相关类别
應用
六水合氯化铜(II)可作为催化剂用于:
- 通过与苯肼、水合肼和取代的芳基异硫氰酸酯反应,将二美酮转化为 N-芳基肼碳硫酰胺基茚唑衍生物。
- 在联吡啶配体存在下,芳基硼酸、芳基氰胺和胺的三组分多米诺反应合成 2-氨基苯并咪唑。
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2
儲存類別代碼
8B - Non-combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Interaction of hydrazine with copper (II) chloride in acidic solutions. Formation, spectral and magnetic properties, and structures of copper (II), copper (I), and mixed-valence species.
Brown DB, et al.
Inorganic Chemistry, 18(10), 2635-2641 (1979)
An X-ray refinement of the crystal structure of copper (II) chloride dihydrate.
Engberg ?.
Acta Chemica Scandinavica, 24(10), 6-6 (1970)
Deprotection of t-butyldimethylsiloxy (TBDMS) protecting group with catalytic copper (II) chloride dihydrate.
TAN ZP, et al.
Chinese Chemical Letters = Zhongguo Hua Xue Kuai Bao, 11(9), 753-756 (2000)
Regeneration of carbonyl compounds from semicarbazones by copper (II) chloride dihydrate.
Ram RN and Varsha K.
Tetrahedron Letters, 32(41), 5829-5832 (1991)
María S Islas et al.
Inorganic chemistry, 53(11), 5724-5737 (2014-05-16)
A new Cu(II) complex with the antihypertensive drug telmisartan, [Cu8Tlm16]·24H2O (CuTlm), was synthesized and characterized by elemental analysis and electronic, FTIR, Raman and electron paramagnetic resonance spectroscopy. The crystal structure (at 120 K) was solved by X-ray diffraction methods. The
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门