推荐产品
等級
pharmaceutical primary standard
API 家族
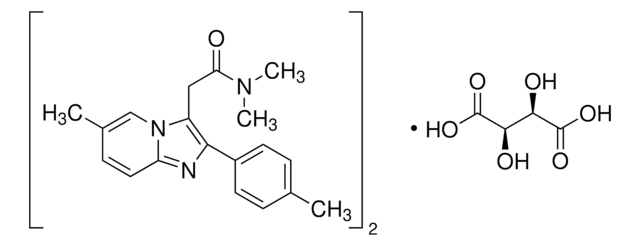
zolpidem
製造商/商標名
EDQM
藥物控制
regulated under CDSA - not available from Sigma-Aldrich Canada; psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
應用
pharmaceutical (small molecule)
格式
neat
InChI
1S/C19H21N3O.C4H6O6/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19;5-1(3(7)8)2(6)4(9)10/h5-10,12H,11H2,1-4H3;1-2,5-6H,(H,7,8)(H,9,10)/t;1-,2-/m.1/s1
InChI 密鑰
NYVVVBWEVRSKIU-LREBCSMRSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Zolpidem tartrate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 2 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Xueyi Feng et al.
Drug testing and analysis, 11(7), 1076-1082 (2019-03-27)
Oral fluid zolpidem detection in the settings of drug-facilitated crime and roadside drug testing indicates recent zolpidem intake. Zolpidem pharmacokinetics in classical biological matrices such as blood and urine have been described; however, reports of such data based on oral
Marieke De Boeck et al.
Journal of pharmaceutical and biomedical analysis, 164, 57-61 (2018-10-22)
The analysis of biological samples, such as whole blood, comes with several sample preparation challenges. Biological matrices often contain a variety of endogenous components that can interfere with the determination of xenobiotics. Especially blood plasma proteins (e.g. serum albumin) are
Marieke De Boeck et al.
Forensic science international, 274, 44-54 (2017-01-18)
To date, thorough clean-up of complex biological samples remains an essential part of the analytical process. The solid phase extraction (SPE) technique is the well-known standard, however, its main weaknesses are the labor-intensive and time-consuming protocols. In this respect, dispersive
Xueyi Feng et al.
Journal of analytical toxicology, 41(9), 735-743 (2017-10-07)
A rapid, selective and sensitive LC-MS-MS method with a post-column addition of acetonitrile was developed and fully validated for the quantitative determination of zolpidem and its major metabolite, zolpidem phenyl-4-carboxylic acid (ZPCA), in oral fluid. Preliminary sample treatment was limited
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门