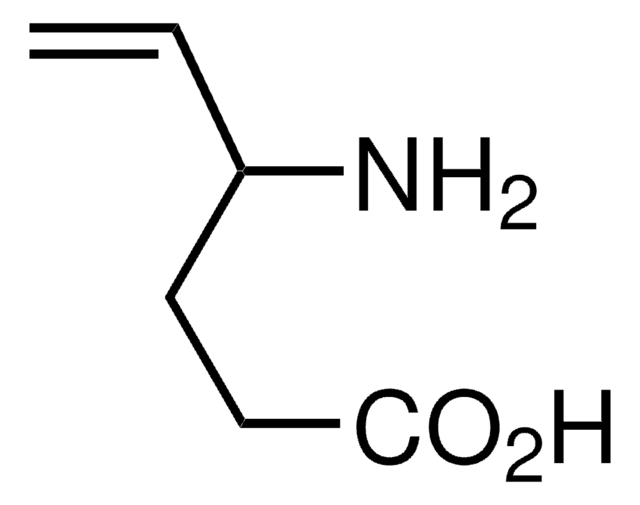
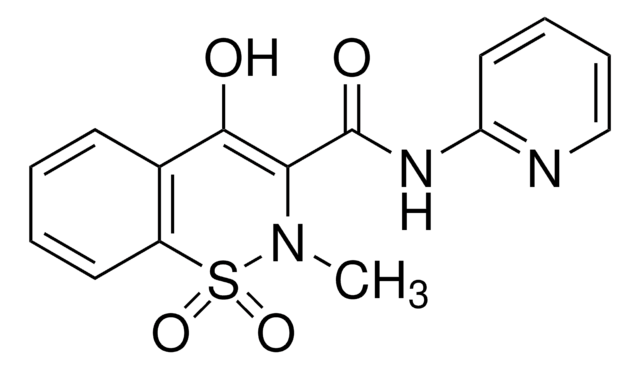
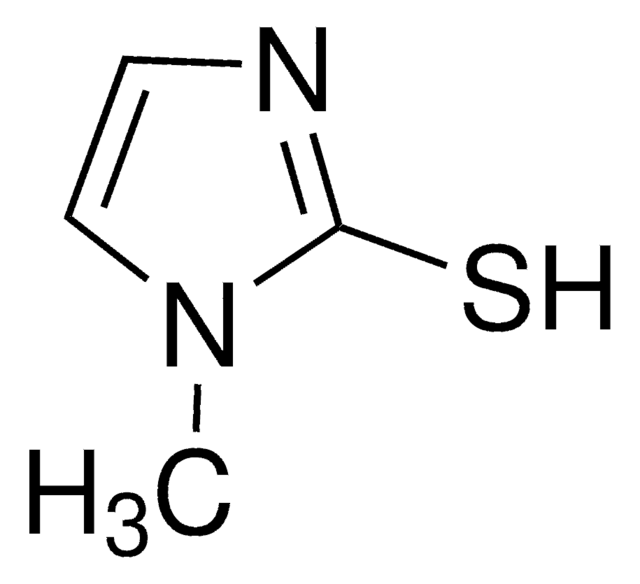
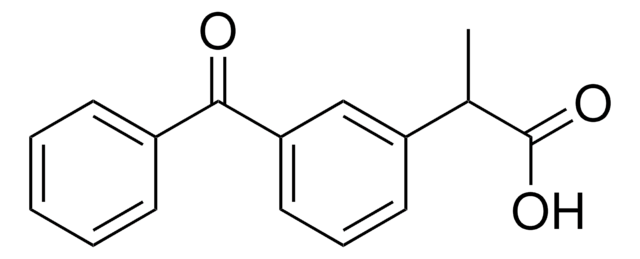
推荐产品
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Vigabatrin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
不可逆的 GABA 转氨酶抑制剂。增加神经末梢的胞内 GABA 浓度;具有抗癫痫活性。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
STOT RE 1
標靶器官
Eyes,Central nervous system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Danièle Bentué-Ferrer et al.
Therapie, 65(1), 23-27 (2010-03-09)
Vigabatrin is a second generation anticonvulsant drug available in France since 1995. It is an amino acid analogue of the GABA, marketed under the racemic form [R(-)/S(+)50/50], but only the S(+)-enantiomer is active. Neither the mechanism of action of vigabatrin
E Ben-Menachem
Acta neurologica Scandinavica. Supplementum, (192)(192), 5-15 (2011-11-09)
Discovered more than three decades ago, vigabatrin is approved in more than 50 countries as adjunctive therapy for adult patients with refractory complex partial seizures who have responded inadequately to several alternative treatments and as monotherapy for pediatric patients aged
Justin A Tolman et al.
Expert opinion on pharmacotherapy, 10(18), 3077-3089 (2009-12-04)
Vigabatrin (Sabril) was approved in the USA in mid-2009 for the adjunctive treatment of refractory complex partial seizures and as treatment of infantile spasms. Vigabatrin's more than 30-year history of research and development is condensed into a clinically relevant review
S D Walker et al.
Acta neurologica Scandinavica. Supplementum, (192)(192), 72-82 (2011-11-09)
Vigabatrin is an effective antiepileptic drug (AED) for the treatment of refractory complex partial seizures (rCPS) and infantile spasms (IS). In clinical trials, vigabatrin was generally well-tolerated with an adverse event profile similar to that of other AEDs. The most
J M Pellock
Acta neurologica Scandinavica. Supplementum, (192)(192), 83-91 (2011-11-09)
Vigabatrin is an effective and well-tolerated antiepileptic drug (AED) for the treatment of refractory complex partial seizures (rCPS) and infantile spasms (IS), but its benefits must be evaluated in conjunction with its risk of retinopathy with the development of peripheral
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门