推荐产品
等級
pharmaceutical primary standard
API 家族
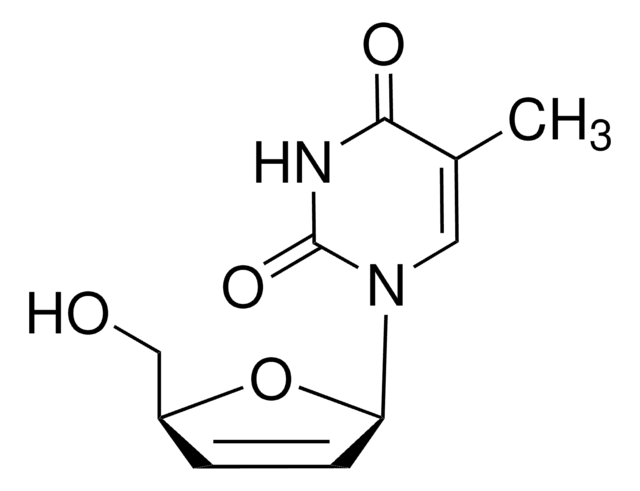
stavudine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
CC1=CN([C@@H]2O[C@H](CO)C=C2)C(=O)NC1=O
InChI
1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1
InChI 密鑰
XNKLLVCARDGLGL-JGVFFNPUSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Stavudine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
A P Lea et al.
Drugs, 51(5), 846-864 (1996-05-01)
Stavudine is a nucleoside analogue which undergoes intracellular phosphorylation to its active metabolite, stavudine-5'-triphosphate. At clinically relevant concentrations, the active metabolite restricts HIV replication by inhibiting the inclusion of thymidine-5'-triphosphate into proviral DNA by HIV reverse transcriptase, and/or by causing
Dam Anh Tran et al.
Sexually transmitted infections, 90(7), 538-544 (2014-03-13)
This study examines the proportions and causes of virological failure after one year of antiretroviral therapy (ART) among people living with HIV (PLHIV) in Vietnam. It also evaluates the positive predictive value (PPV) of immunological criteria to detect treatment failure.
R L Murphy
Antiviral therapy, 3 Suppl 4, 69-73 (2000-03-21)
Initial studies of multiple-agent antiretroviral combinations including the thymidine nucleoside analogue reverse transcriptase inhibitor (RTI) stavudine (2',3'-didehydro-2',3'-dideoxythymidine; D4T) have shown potent anti-HIV effects in both treatment-naive and -experienced patients. A number of ongoing randomized comparative trials are assessing stavudine-based multiple
Nilza Nascimento Guimarães et al.
Expert opinion on drug safety, 9(5), 771-781 (2010-04-10)
The nucleoside reverse transcriptase inhibitors (NRTIs) are used in antiretroviral therapy worldwide for the treatment of HIV infections. These drugs act by blocking reverse transcriptase enzyme activity, causing pro-viral DNA chain termination. As a consequence, NRTIs could cause genomic instability
G Skowron
The Journal of infectious diseases, 171 Suppl 2, S113-S117 (1995-03-01)
Data on the biologic effects and safety of stavudine in patients with AIDS and AIDS-related complex represent results of two phase I trials (n = 84), another phase I study of patients with hematologic intolerance to zidovudine (n = 23)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门