PHR1987
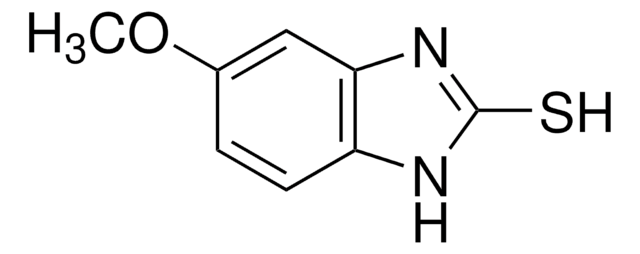
奥美拉唑杂质B
Pharmaceutical Secondary Standard; Certified Reference Material
别名:
2-[(RS)-[(3,5-Dimethylpyridin-2-yl)methyl]sulfinyl]-5-methoxy-1H-benzimidazole, 2-[(RS)-[(3,5-dimethylpyridin-2-yl)methyl]sulphinyl]-5-methoxy-1h-benzimidazole, 2-[[(3,5-Dimethyl-2-pyridinyl)methyl]sulfinyl]--6-methoxy-1H-benzimidazol, 4-Desmethoxy Omeprazole
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
API 家族
omeprazole
CofA
current certificate can be downloaded
包裝
pkg of 30 mg
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
InChI
1S/C16H17N3O2S/c1-10-6-11(2)15(17-8-10)9-22(20)16-18-13-5-4-12(21-3)7-14(13)19-16/h4-8H,9H2,1-3H3,(H,18,19)
InChI 密鑰
ZMXZYNHJPJEPAE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Omeprazole Impurity B is found to be as an impurity of omeprazole. Omeprazole is a benzimidazole derivative, which is widely used as an antiulcer drug and against other acid-related diseases.
應用
Olmesartan medoxomil may be used as a pharmaceutical secondary standard for the determination of the analyte in pharmaceutical formulations using different analytical techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAB4099 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Extractive spectrophotometric determination of omeprazole in pharmaceutical preparations
Bhandage A, et al.
Tropical Journal of Pharmaceutical Research, 8(5), 544-549 (2009)
A validated normal phase HPLC method for simultaneous determination of drotaverine hydrochloride and omeprazole in pharmaceutical formulation
Topagi SK, et al.
Asian Journal of Pharmaceutical and Clinical Research, 3(1), 20-24 (2010)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门