PHR1608
Lactulose
Pharmaceutical Secondary Standard; Certified Reference Material
别名:
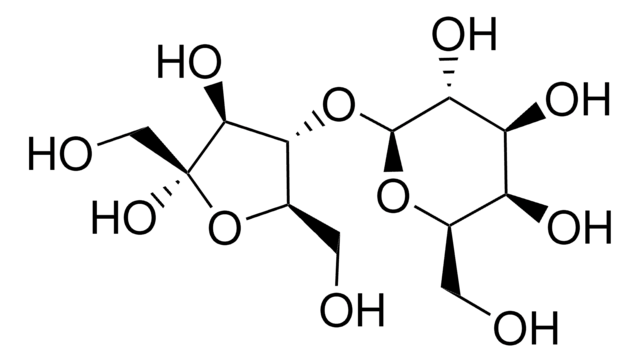
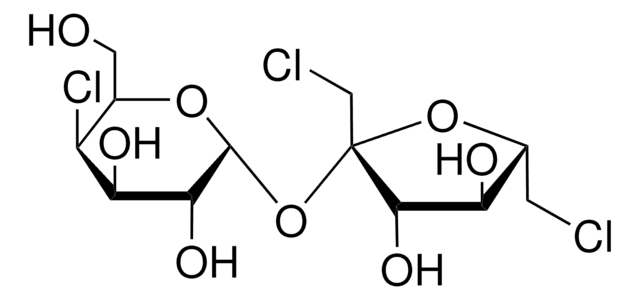
Lactulose, 4-O-β-D-Galactopyranosyl-D-fructofuranose, 4-O-β-D-Galactopyranosyl-D-fructose
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C12H22O11
CAS号:
分子量:
342.30
Beilstein:
93773
EC號碼:
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to BP 210
traceable to Ph. Eur. L0130000
traceable to USP 1356803
API 家族
lactulose
CofA
current certificate can be downloaded
包裝
pkg of 1 g
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
OC[C@H]1O[C@@H](O[C@@H]2[C@@H](CO)O[C@@](O)(CO)[C@H]2O)[C@H](O)[C@@H](O)[C@H]1O
InChI
1S/C12H22O11/c13-1-4-6(16)7(17)8(18)11(21-4)22-9-5(2-14)23-12(20,3-15)10(9)19/h4-11,13-20H,1-3H2/t4-,5-,6+,7+,8-,9-,10+,11+,12+/m1/s1
InChI 密鑰
JCQLYHFGKNRPGE-WJONTELPSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Lactulose is a disaccharide that finds immense applications in medicine, nutrition and dairy technology. It is utilized as a medicine in the treatment of portal systemic encephalopathy and chronic constipation.
Lactulose is a disaccharide that finds immense applications in medicine, nutrition and dairy technology. It is utilized as a medicine in the treatment of portal systemic encephalopathy and chronic constipation.
應用
Lactulose may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by various chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAB7605 in the slot below. This is an example certificate only and may not be the lot that you receive.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Chromatographic determination of lactulose
Martinez-Castro I, et al.
Chromatographia, 23(2), 132-136 (1987)
Use of an automated robotic system for the extraction and on-line high-performance liquid chromatographic analysis of pharmaceutical formulations
Van der Voorden EC, et al.
Chemometrics and Intelligent Laboratory Systems, 17(1), 129-135 (1992)
Kostan W Reisinger et al.
Journal of pediatric gastroenterology and nutrition, 59(6), 720-724 (2014-08-12)
The incidence of necrotizing enterocolitis (NEC) is higher in formula-fed babies than in breast-fed babies, which may be caused by breast-feeding-induced gut maturation. The effect of breast-feeding on gut maturation has been widely studied in animal models. This study aimed
A A Guerra-Ordaz et al.
Applied and environmental microbiology, 80(16), 4879-4886 (2014-06-08)
The potential of a prebiotic oligosaccharide lactulose, a probiotic strain of Lactobacillus plantarum, or their synbiotic combination to control postweaning colibacillosis in pigs was evaluated using an enterotoxigenic Escherichia coli (ETEC) K88 oral challenge. Seventy-two weanlings were fed four diets:
Jun Fang et al.
Journal of pharmaceutical sciences, 103(10), 3235-3243 (2014-07-22)
Bacteria of micrometer size could accumulate in tumor based on enhanced permeability and retention (EPR) effect. We report here Lactobacillus casei (L. casei), a nonpathogenic facultatively anaerobic bacterium, preferentially accumulated in tumor tissues after intravenously (i.v.) injection; at 24 h
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门