推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1526007
API 家族
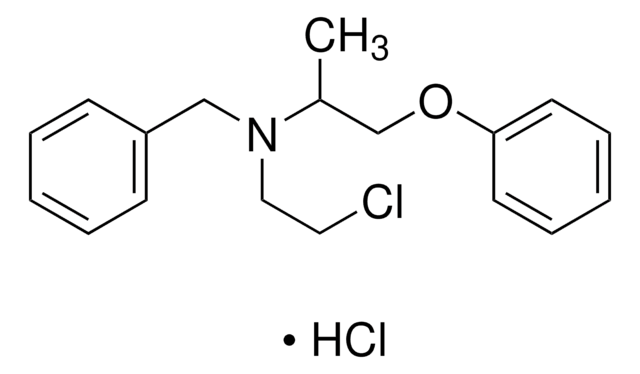
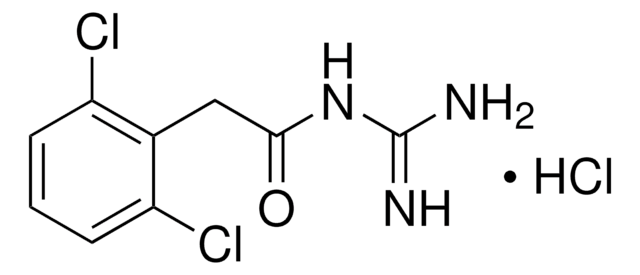
phenoxybenzamine
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-30°C
SMILES 字串
Cl[H].CC(COc1ccccc1)N(CCCl)Cc2ccccc2
InChI
1S/C18H22ClNO.ClH/c1-16(15-21-18-10-6-3-7-11-18)20(13-12-19)14-17-8-4-2-5-9-17;/h2-11,16H,12-15H2,1H3;1H
InChI 密鑰
VBCPVIWPDJVHAN-UHFFFAOYSA-N
基因資訊
human ... ADRA1A(148) , ADRA1B(147) , ADRA1D(146) , ADRA2A(150) , ADRA2B(151) , ADRA2C(152)
正在寻找类似产品? 访问 产品对比指南
一般說明
Phenoxybenzamine Hydrochloride is an important drug belonging to the class of β-chloroethylamine drugs. It functions as an α-blocker by binding to α-adrenoreceptors, located on vascular smooth muscles and stops the influence of sympathetic nerves on blood vessels. It is widely used in the treatment of hypertension, benign prostatic hyperplasia and hypoplastic left heart syndrome.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
Phenoxybenzamine Hydrochloride may be used as a pharmaceutical reference standard in the determination of the analyte in pharmaceutical formulations by chromatographic and chemiluminescence techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
生化/生理作用
钙调蛋白拮抗剂;α-肾上腺素受体拮抗剂。
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA2196 in the slot below. This is an example certificate only and may not be the lot that you receive.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Sensitive chemiluminescence determination of phentolamine mesylate and phenoxybenzamine hydrochloride based on K3Fe (CN) 6-H2O2-fluorescein
Xing L, et al.
Journal of Luminescence, 137(38), 162-167 (2013)
Quantitative gas chromatographic determination of azapetine and phenoxybenzamine in pharmaceutical preparations
Silvestri S and Taponeco G
Journal of Chromatography A, 37(38), 200-204 (1968)
A facile enantioselective synthesis of enantiomerically pure (R)-phenoxybenzamine hydrochloride using the hydrolytic kinetic resolution method
Nikalje MD, et al.
Tetrahedron Asymmetry, 21(23), 2825-2829 (2010)
Chiral aziridine ring opening: facile synthesis of (R)-mexiletine and (R)-phenoxybenzamine hydrochloride
Viswanadh N, et al.
Tetrahedron Letters, 56(38), 5269-5271 (2015)
Phenoxybenzamine (hydrochloride).
IARC monographs on the evaluation of the carcinogenic risk of chemicals to man, 9, 223-228 (1975-01-01)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门