推荐产品
生物源
synthetic
等級
pharmaceutical primary standard
agency
EP
API 家族
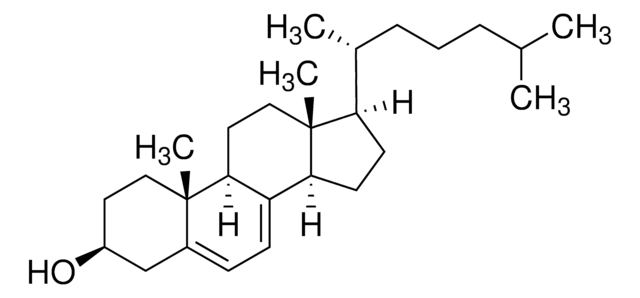
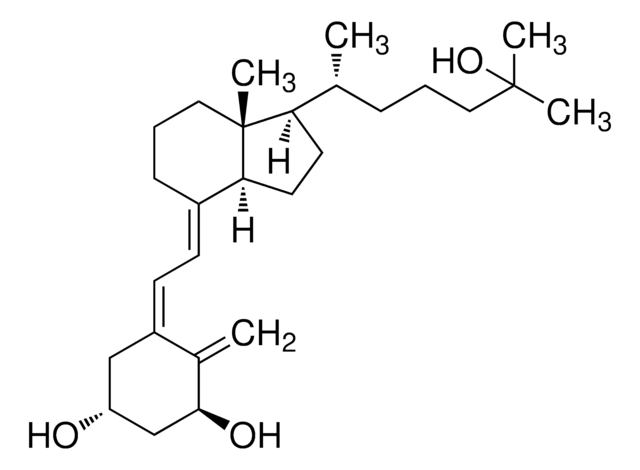
cholecalciferol
形狀
solid
製造商/商標名
EDQM
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
mp
83-86 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
−20°C
SMILES 字串
CC(C)CCC[C@@H](C)[C@@]1([H])CC[C@@]([C@]1(C)CCC/2)([H])C2=C\C=C(C[C@@H](O)CC3)/C3=C
InChI
1S/C27H44O/c1-19(2)8-6-9-21(4)25-15-16-26-22(10-7-17-27(25,26)5)12-13-23-18-24(28)14-11-20(23)3/h12-13,19,21,24-26,28H,3,6-11,14-18H2,1-2,4-5H3/b22-12+,23-13-/t21-,24+,25-,26+,27-/m1/s1
InChI 密鑰
QYSXJUFSXHHAJI-YRZJJWOYSA-N
基因資訊
human ... VDR(7421)
正在寻找类似产品? 访问 产品对比指南
一般說明
Cholecalciferol, also referred to as vitamin D3, promotes the absorption of calcium and phosphorus in the body to regulate bone growth. It is most commonly found in humans and animals and is produced in our skin when exposed to sunlight. It is used to treat and prevent bone disorders (such as rickets, osteomalacia).
應用
生化/生理作用
包裝
其他說明
相關產品
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - STOT RE 1 Oral
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门