推荐产品
等級
pharmaceutical primary standard
API 家族
busulfan
製造商/商標名
EDQM
mp
114-117 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
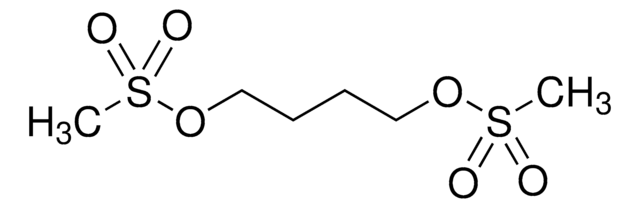
CS(=O)(=O)OCCCCOS(C)(=O)=O
InChI
1S/C6H14O6S2/c1-13(7,8)11-5-3-4-6-12-14(2,9)10/h3-6H2,1-2H3
InChI 密鑰
COVZYZSDYWQREU-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Carc. 1A - Muta. 1B - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Ibrahim El-Serafi et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 913-914, 98-105 (2013-01-05)
Busulphan is an alkylating agent used as conditioning regimen prior to stem cell transplantation. Busulphan is metabolized in the liver and four major metabolites have been identified. The first metabolite is tetrahydrothiophene which is oxidized to tetrahydrothiophene 1-oxide, then sulfolane
Arnon Nagler et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31(28), 3549-3556 (2013-08-28)
Cyclophosphamide (Cy) combined with total-body irradiation (TBI) or with busulfan (Bu) are currently the most common myeloablative regimens used in allogeneic stem-cell transplantation (alloSCT) in adults with acute myelogenous leukemia (AML). Intravenous (IV) Bu has more predictable bioavailability and a
Je-Hwan Lee et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31(6), 701-709 (2012-11-07)
We conducted a phase III randomized clinical trial to compare two myeloablative conditioning regimens for allogeneic hematopoietic cell transplantation (HCT) in patients with leukemia and myelodysplastic syndrome. After randomization, 64 patients received busulfan (3.2 mg/kg per day × 4 days)
Edward A Copelan et al.
Blood, 122(24), 3863-3870 (2013-09-26)
Cyclophosphamide combined with total body irradiation (Cy/TBI) or busulfan (BuCy) are the most widely used myeloablative conditioning regimens for allotransplants. Recent data regarding their comparative effectiveness are lacking. We analyzed data from the Center for International Blood and Marrow Transplant
Christopher Bredeson et al.
Blood, 122(24), 3871-3878 (2013-10-02)
We conducted a prospective cohort study testing the noninferiority of survival of ablative intravenous busulfan (IV-BU) vs ablative total body irradiation (TBI)-based regimens in myeloid malignancies. A total of 1483 patients undergoing transplantation for myeloid malignancies (IV-BU, N = 1025;
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门