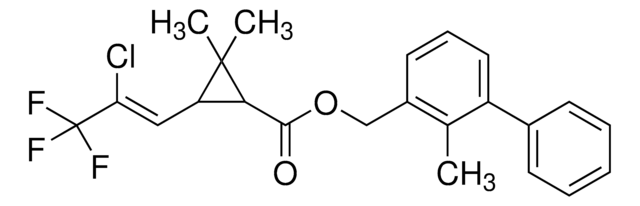
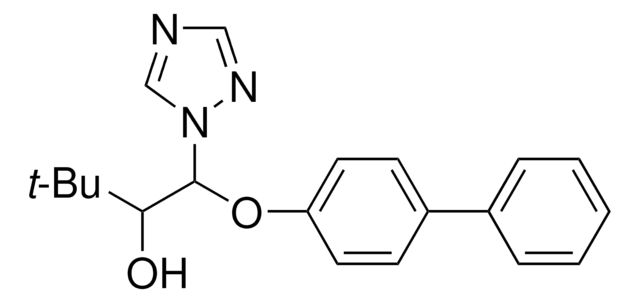
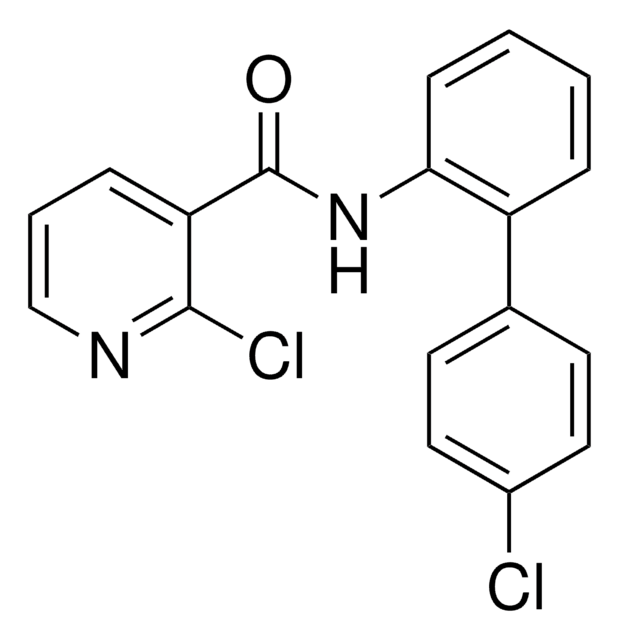
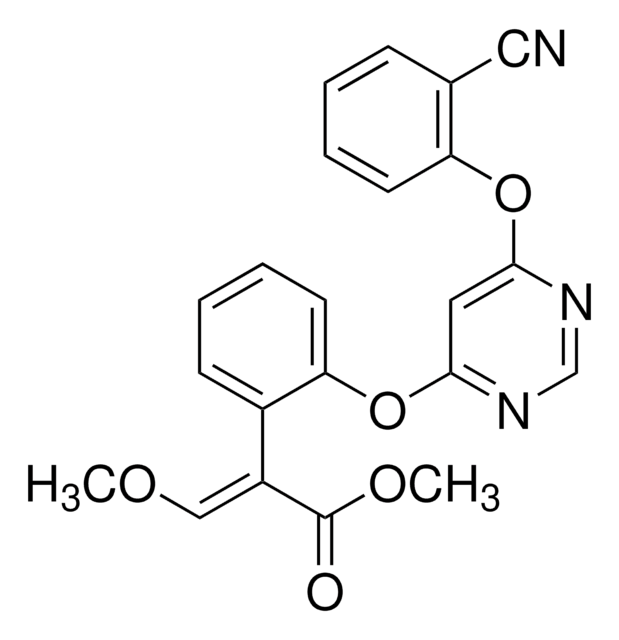
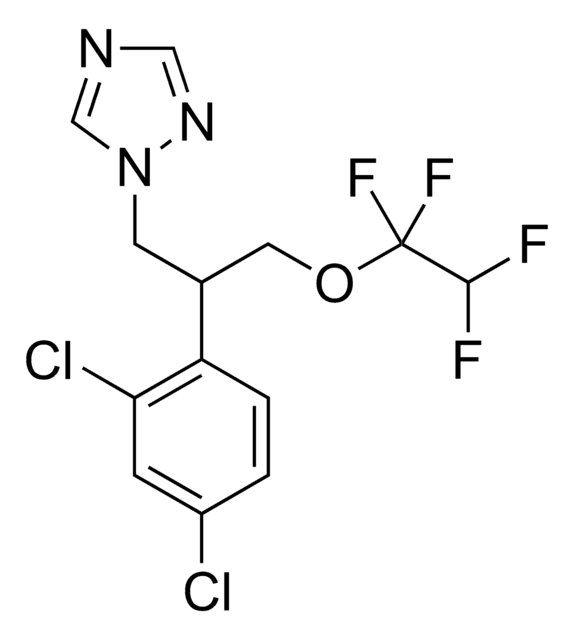
推荐产品
等級
analytical standard
品質等級
描述
mixture of stereo isomers
產品線
PESTANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
agriculture
environmental
形式
neat
SMILES 字串
CC(C)(C)C(O)C(Oc1ccc(cc1)-c2ccccc2)n3cncn3
InChI
1S/C20H23N3O2/c1-20(2,3)18(24)19(23-14-21-13-22-23)25-17-11-9-16(10-12-17)15-7-5-4-6-8-15/h4-14,18-19,24H,1-3H3
InChI 密鑰
VGPIBGGRCVEHQZ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
法律資訊
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
未找到合适的产品?
试试我们的产品选型工具.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
No data available
閃點(°C)
No data available
Tatiana Zamora et al.
Journal of separation science, 27(9), 645-652 (2004-09-25)
Coupled-column liquid chromatography with fluorescence detection was applied to the determination of o-phenylphenol and bitertanol residues in orange and banana fruits. After extraction with a mixture of acetone, dichloromethane-petroleum ether, and ethyl acetate, an extract aliquot of 100 microL was
Y Yamazaki et al.
Journal of AOAC International, 81(6), 1252-1256 (1998-12-16)
A simple and rapid method was developed for determining bitertanol residues in strawberries. Bitertanol was extracted from samples with ethyl acetate. Bitertanol acetate was added prior to extraction as a surrogate standard. The ethyl acetate extract was cleaned up by
Marc Bourgin et al.
Chemosphere, 90(4), 1387-1395 (2012-09-25)
The degradation of bitertanol by ozone treatment is investigated. Solutions of bitertanol (8.4 μg mL(-1)) were prepared either by dissolution of the standard or by dilution of Gaucho Blé seed loading solution and then ozonated under different conditions. Evolution of
Ping-Kun Chan et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 44(12), 2047-2057 (2006-09-15)
The effects of fungicide bitertanol on cytochrome P450-dependent monooxygenases were studied using rats treated intraperitoneally with the N-substituted triazole for 4 days. Treatment with 10, 25, and 100 mg/kg bitertanol produced 2-, 4-, and 14-fold increases of 7-ethoxyresorufin O-deethylation activity
A R Allen et al.
Neurotoxicology and teratology, 15(4), 237-242 (1993-07-01)
Several recent reports indicate that triadimefon, a triazole fungicide, has effects on behavior that are similar to those of psychomotor stimulants. For example, triadimefon increases overall fixed-interval (FI) response rate, disrupts FI response patterning, increases motor activity, and produces stereotypies
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门