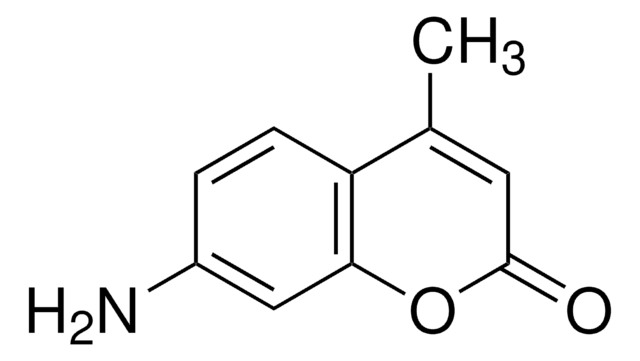
推荐产品
等級
primary reference standard
蒸汽壓力
0.01 mmHg ( 47 °C)
儲存期限
limited shelf life, expiry date on the label
製造商/商標名
HWI
bp
298 °C (lit.)
mp
68-73 °C (lit.)
應用
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
SMILES 字串
O=C1Oc2ccccc2C=C1
InChI
1S/C9H6O2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H
InChI 密鑰
ZYGHJZDHTFUPRJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Produced and qualified by HWI pharma services GmbH.
Exact content by quantitative NMR can be found on the certificate.
Exact content by quantitative NMR can be found on the certificate.
應用
草药产品分析的标准品。
其他說明
This compound is commonly found in plants of the genus: cinnamomum curcuma hedera humulus melissa mentha salvia sambucus zingiber
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Skin Sens. 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 1
閃點(°F)
323.6 °F - closed cup
閃點(°C)
162 °C - closed cup
历史批次信息供参考:
Jack Cazes
Encyclopedia of Chromatography, 206-206 (2001)
The pharmacology, metabolism, analysis, and applications of coumarin and coumarin-related compounds.
D Egan et al.
Drug metabolism reviews, 22(5), 503-529 (1990-01-01)
Musiliyu A Musa et al.
Current medicinal chemistry, 15(26), 2664-2679 (2008-11-11)
The coumarin (benzopyran-2-one, or chromen-2-one) ring system, present in natural products (such as the anticoagulant warfarin) that display interesting pharmacological properties, has intrigued chemists and medicinal chemists for decades to explore the natural coumarins or synthetic analogs for their applicability
Klaus Abraham et al.
Molecular nutrition & food research, 54(2), 228-239 (2009-12-22)
Coumarin is a secondary phytochemical with hepatotoxic and carcinogenic properties. For the carcinogenic effect, a genotoxic mechanism was considered possible, but was discounted by the European Food Safety Authority in 2004 based on new evidence. This allowed the derivation of
Sukhendu Maiti et al.
Journal of the American Chemical Society, 135(11), 4567-4572 (2013-03-07)
We present here, the design, synthesis, spectroscopic characterization, and in vitro biological assessment of a gemcitabine-coumarin-biotin conjugate (5). Probe 5 is a multifunctional molecule composed of a thiol-specific cleavable disulfide bond, a coumarin moiety as a fluorescent reporter, gemcitabine (GMC)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门