About This Item
推荐产品
等級
primary reference standard
儲存期限
limited shelf life, expiry date on the label
製造商/商標名
HWI
mp
222 °C (dec.) (lit.)
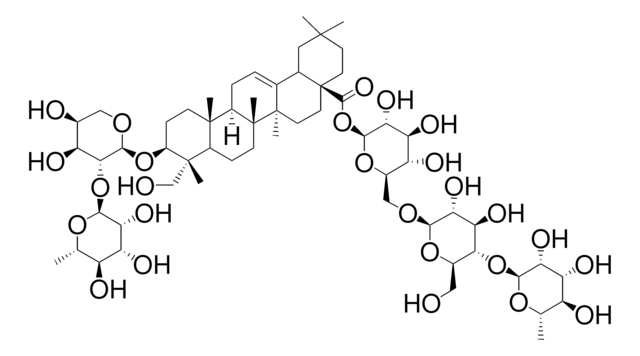
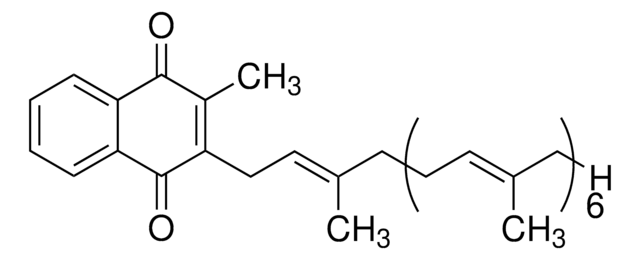
SMILES 字串
[H][C@]12CC=C3C4CC(C)(C)CC[C@@]4(CC[C@@]3(C)[C@]1(C)CCC5[C@](C)(CO)[C@H](CC[C@]25C)O[C@@H]6OC[C@H](O)[C@H](O)[C@H]6O[C@@H]7O[C@@H](C)[C@H](O)[C@@H](O)[C@H]7O)C(=O)O[C@@H]8O[C@H](CO[C@@H]9O[C@H](CO)[C@@H](O[C@@H]%10O[C@@H](C)[C@H](O)[C@@H](O)[C@H]%10O)[C@H](O)[C@H]9O)[C@@H](O)[C@H](O)[C@H]8O
InChI
1S/C59H96O26/c1-24-34(63)38(67)42(71)49(78-24)83-46-29(20-60)80-48(45(74)41(46)70)77-22-30-37(66)40(69)44(73)51(81-30)85-53(75)59-17-15-54(3,4)19-27(59)26-9-10-32-55(5)13-12-33(56(6,23-61)31(55)11-14-58(32,8)57(26,7)16-18-59)82-52-47(36(65)28(62)21-76-52)84-50-43(72)39(68)35(64)25(2)79-50/h9,24-25,27-52,60-74H,10-23H2,1-8H3/t24-,25-,27?,28-,29+,30+,31?,32+,33-,34-,35-,36-,37+,38+,39+,40-,41+,42+,43+,44+,45+,46+,47+,48+,49-,50-,51-,52-,55-,56-,57+,58+,59-/m0/s1
InChI 密鑰
RYHDIBJJJRNDSX-WBOYGZCJSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Exact content by quantitative NMR can be found on the certificate.
應用
其他說明
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门