推荐产品
product name
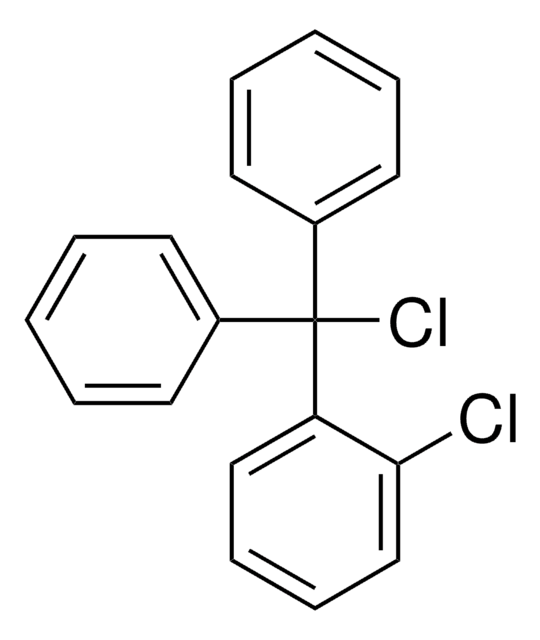
2-Chlorotrityl chloride resin (100-200 mesh), 1% DVB, Novabiochem®
品質等級
產品線
Novabiochem®
形狀
beads
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
應用
peptide synthesis
儲存溫度
2-30°C
一般說明
2-Chlorotrityl chloride resin is an extremely versatile, acid-labile resin for the solid phase immobilization of carboxylic acids [1,2,3,4,5,6, 20], alcohols [7,8,9, 20], phenols [10,11,12,13, 20] and amines [14,15,16,17,18,19,20], imidazoles [20] and hydroxylamines [21,22]. Release of these functionalities is generally achieved using 1-50% TFA in DCM containing 5% TIS, although carboxylic acids can also be cleaved from this support with AcOH/TFE/DCM [1,2,3,4,5,6],0.5% TFA in DCM, or HFIP in DCM [23].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis ResinsLiterature references
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3943.
[3] K. Barlos, et al. (1991) Angew. Chem. Int. Ed. Engl., 30, 590.
[4] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 37, 513.
[5] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 562.
[6] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 555.
[7] H. Wenschuh, et al. in ′Peptides 1994, Proc. 23rd European Peptide Symposium′, H. Maia (Eds), ESCOM, Leiden, 1995, pp. 287.
[8] H. Wenschuh, et al. (1995) J. Org. Chem., 60, 405.
[9] B. B. Shankar, et al. (1998) Tetrahedron Lett., 39, 2447.
[10] Z. Zhu & B. Mckittrick (1998) Tetrahedron Lett., 39, 7479.
[11] U. Heinelt, et al. (2001) Bioorg. Med. Chem. Lett., 11, 227.
[12] K. J. Elgie, et al. (2000) Tetrahedron Lett., 41, 2753.
[13] S. Batra, et al. (2000) Tetrahedron Lett., 41, 5971.
[14] M. Cardno, et al. (1995) J. Chem. Soc., Chem. Commun., 2163.
[15] I. A. Nash, et al. (1996) Tetrahedron Lett., 37, 2625.
[16] W. J. Hoekstra, et al. (1997) Tetrahedron Lett., 38, 2629.
[17] J. Perumattan, et al. (1998) Mol. Div., 3, 121.
[18] J. J. McNally, et al. (1998) Tetrahedron Lett., 39,967.
[19] M. A. Youngman & S. L. Dax (1997) Tetrahedron Lett., 38, 6347.
[20] A. Bernhardt, et al. (1997) J. Peptide Res., 50, 143.
[21] S. L. Mellor, et al. (1997) Tetrahedron Lett., 38, 3311.
[22] M. M. Meloni & M. Taddei (2001) Org. Lett., 3, 337.
[23] R. Bollhagen, et al. (1994) J. Chem. Soc ., Chem. Commun., 2559.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis ResinsLiterature references
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3943.
[3] K. Barlos, et al. (1991) Angew. Chem. Int. Ed. Engl., 30, 590.
[4] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 37, 513.
[5] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 562.
[6] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 555.
[7] H. Wenschuh, et al. in ′Peptides 1994, Proc. 23rd European Peptide Symposium′, H. Maia (Eds), ESCOM, Leiden, 1995, pp. 287.
[8] H. Wenschuh, et al. (1995) J. Org. Chem., 60, 405.
[9] B. B. Shankar, et al. (1998) Tetrahedron Lett., 39, 2447.
[10] Z. Zhu & B. Mckittrick (1998) Tetrahedron Lett., 39, 7479.
[11] U. Heinelt, et al. (2001) Bioorg. Med. Chem. Lett., 11, 227.
[12] K. J. Elgie, et al. (2000) Tetrahedron Lett., 41, 2753.
[13] S. Batra, et al. (2000) Tetrahedron Lett., 41, 5971.
[14] M. Cardno, et al. (1995) J. Chem. Soc., Chem. Commun., 2163.
[15] I. A. Nash, et al. (1996) Tetrahedron Lett., 37, 2625.
[16] W. J. Hoekstra, et al. (1997) Tetrahedron Lett., 38, 2629.
[17] J. Perumattan, et al. (1998) Mol. Div., 3, 121.
[18] J. J. McNally, et al. (1998) Tetrahedron Lett., 39,967.
[19] M. A. Youngman & S. L. Dax (1997) Tetrahedron Lett., 38, 6347.
[20] A. Bernhardt, et al. (1997) J. Peptide Res., 50, 143.
[21] S. L. Mellor, et al. (1997) Tetrahedron Lett., 38, 3311.
[22] M. M. Meloni & M. Taddei (2001) Org. Lett., 3, 337.
[23] R. Bollhagen, et al. (1994) J. Chem. Soc ., Chem. Commun., 2559.
應用
- Efficient Synthesis of Protein Mimics by Sequential Native Chemical Ligation: This study utilized 2-Chlorotrityl chloride resin (100-200 mesh, 1% DVB) for solid-phase peptide synthesis, highlighting its effective use in complex peptide assembly processes. (Kruijtzer & Liskamp).
聯結
Replaces: 01-64-0114
分析報告
Colour (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Ala-Leu loaded resin): 1.00 - 1.80 mmol/g
Swelling Volume (in CH₂Cl₂): 2.0 - 5.0 ml/g
Total swelling volume acc. Houben Weyl (in CH2Cl2): ≥ 4.2 ml/g
The polymer matrix is copoly (styrene-1 % DVB) 100 - 200 mesh.
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Ala-Leu loaded resin): 1.00 - 1.80 mmol/g
Swelling Volume (in CH₂Cl₂): 2.0 - 5.0 ml/g
Total swelling volume acc. Houben Weyl (in CH2Cl2): ≥ 4.2 ml/g
The polymer matrix is copoly (styrene-1 % DVB) 100 - 200 mesh.
法律資訊
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
商品
Novabiochem® has one of the most extensive ranges of linkers and derivatized resins for Fmoc solid phase peptide synthesis. These resins have varied properties with special protocols for loading and cleaving.
Novabiochem® has one of the most extensive ranges of linkers and derivatized resins for Fmoc solid phase peptide synthesis. These resins have varied properties with special protocols for loading and cleaving.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门