推荐产品
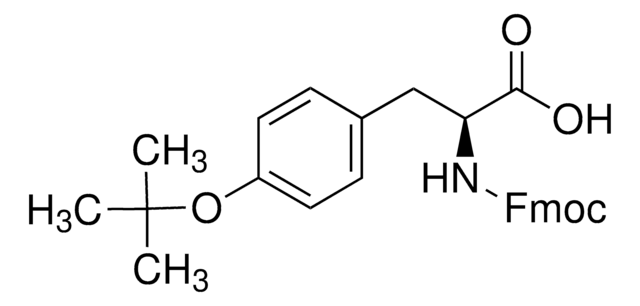
product name
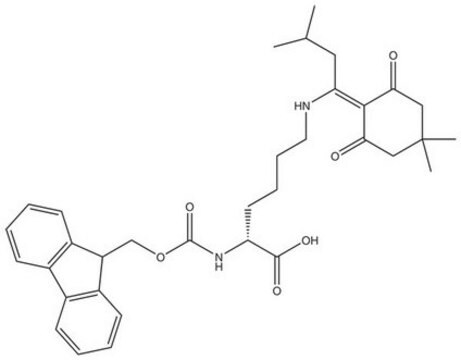
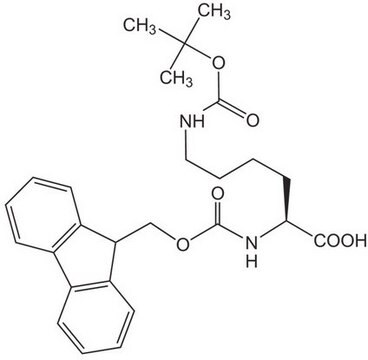
Fmoc-Dpr(ivDde)-OH, Novabiochem®
品質等級
產品線
Novabiochem®
化驗
≥95.0% (acidimetric)
≥98% (TLC)
≥99.0% (HPLC)
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
應用
peptide synthesis
官能基
amine
儲存溫度
15-25°C
InChI
1S/C31H36N2O6/c1-18(2)13-24(28-26(34)14-31(3,4)15-27(28)35)32-16-25(29(36)37)33-30(38)39-17-23-21-11-7-5-9-19(21)20-10-6-8-12-22(20)23/h5-12,18,23,25,32H,13-17H2,1-4H3,(H,33,38)(H,36,37)/t25-/m0/s1
InChI 密鑰
HLIFXCXTXPXGNH-VWLOTQADSA-N
一般說明
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] S. R. Chhabra, et al. (1998) Tetrahedron Lett., 39, 1603.
[2] R. Wilhelm, et al., Poster 34 presented at the 16th American Peptide Symposium, Minneapolis, 1999.
[3] J. Beythien & P. Schneeberger, in ′Peptides 2000, Proc. 26th European Peptide Symposium′, EDK, Paris, 2001, pp. 361.
[4] B. Rohwedder, et al. (1998) Tetrahedron Lett., 39, 1175.
聯結
分析報告
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Purity (TLC(157A)): ≥ 98 %
Purity (TLC(CMA2)): ≥ 98 %
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 95.0 %
Water (K. F.): ≤ 1.00 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
法律資訊
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门