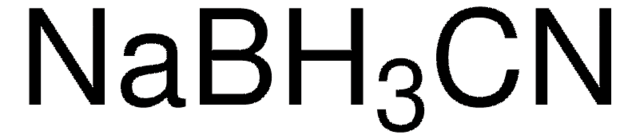推荐产品
蒸汽壓力
<1 hPa ( 25 °C)
品質等級
形狀
solid
自燃溫度
220 °C
效力
160 mg/kg LD50, oral (Rat)
230 mg/kg LD50, skin (Rabbit)
expl. lim.
3.02 % (v/v)
反應適用性
reagent type: reductant
pH值
11 (20 °C, 10 g/L in H2O)
bp
500 °C (decomposition)
mp
>360 °C (slow decomposition)
轉變溫度
flash point 69 °C
密度
1.07 g/cm3 at 20 °C
體積密度
350‑500 kg/m3
儲存溫度
2-30°C
InChI
1S/BH4.Na/h1H4;/q-1;+1
InChI 密鑰
YOQDYZUWIQVZSF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- Zero valent iron nanoparticles supported on mesoporous carbon (Starbon) by in situ reductions of Fe3+ ions.
- Ag nanoparticles on graphene using AgNO3 as a precursor.
- Ag-Pd/ZrO2 catalytic system applicable in plasmonic catalysis. It can also be used as a source to store as well as generate hydrogen for energy-carrier applications.
分析報告
Identity (ICP-OES): passes test
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B - Water-react 1
安全危害
儲存類別代碼
4.3 - Hazardous materials, which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
相关内容
Fmoc resin cleavage and deprotection follows the difficult task of detaching the peptide from the resin support and removing all the side-chain protecting groups of the amino acid residues to yield the desired peptide.
Fmoc resin cleavage and deprotection follows the difficult task of detaching the peptide from the resin support and removing all the side-chain protecting groups of the amino acid residues to yield the desired peptide.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门