推荐产品
product name
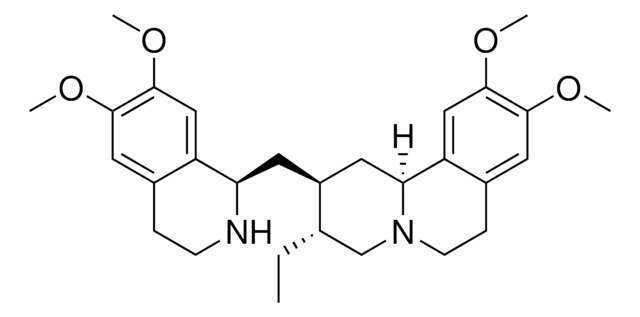
Emetine, Dihydrochloride, Principal alkaloid of ipecac, isolated from the ground roots of Uragoga ipecacuanha.
品質等級
化驗
≥98% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
desiccated (hygroscopic)
protect from light
顏色
white to off-white
white
溶解度
water: 20 mg/mL
ethanol: soluble
運輸包裝
ambient
儲存溫度
2-8°C
InChI
1S/C29H40N2O4.2ClH/c1-6-18-17-31-10-8-20-14-27(33-3)29(35-5)16-23(20)25(31)12-21(18)11-24-22-15-28(34-4)26(32-2)13-19(22)7-9-30-24;;/h13-16,18,21,24-25,30H,6-12,17H2,1-5H3;2*1H/t18-,21-,24+,25-;;/m0../s1
InChI 密鑰
JROGBPMEKVAPEH-GXGBFOEMSA-N
一般說明
Principal alkaloid of ipecac, isolated from the ground roots of Uragoga ipecacuanha. Irreversibly blocks protein synthesis in eukaryotes by inhibiting the movement of ribosomes along the mRNA. Induces hypotension by blocking adrenoreceptors. Interferes with cytometric analysis of DNA replication at the early S phase. Also identified as a specific inhibitor of HIF-2α protein stability and transcriptional activity (IC50 ≤1 µM.
Principle alkaloid of ipecac, isolated from the ground roots of Uragoga ipecacuanha. Irreversibly blocks protein synthesis in eukaryotes by inhibiting the movement of ribosomes along the mRNA. Induces hypotension by blocking adrenoreceptors. Inhibits cytometric analysis of DNA replication at the early S phase. Also identified as a specific inhibitor of HIF-2α protein stability and transcriptional activity (IC50 ≤1 µM.
生化/生理作用
Cell permeable: no
Primary Target
Movement of ribosomes along the mRNA
Movement of ribosomes along the mRNA
Product does not compete with ATP.
Reversible: no
Target IC50: ≤1 µM as a specific inhibitor of HIF-2α protein stability and transcriptional activity
警告
Toxicity: Highly Toxic (H)
重構
Following reconstitution aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
其他說明
Due to the nature of the Hazardous Materials in this shipment, additional shipping charges may be applied to your order. Certain sizes may be exempt from the additional hazardous materials shipping charges. Please contact your local sales office for more information regarding these charges.
Kong, H.S., et al. 2010. Mol. Pharmacol.in press.
Khan, M.A. 1995. Prog. Neurobiol.46, 541.
Kokuho, T., et al. 1995. Immunobiology193, 42.
Lee, Y.S., and Wurster, R.D. 1995. Cancer Lett.93, 157.
Burhans, W.C., et al. 1991. EMBO J. 10, 4351.
Filley, E.A., and Rook, G.A. 1991. Infect. Immun.59, 2567.
Landis, R.C., et al. 1991. J. Immunol.146, 128.
Schweighoffer, T., et al. 1991. Histochemistry96, 93.
Khan, M.A. 1995. Prog. Neurobiol.46, 541.
Kokuho, T., et al. 1995. Immunobiology193, 42.
Lee, Y.S., and Wurster, R.D. 1995. Cancer Lett.93, 157.
Burhans, W.C., et al. 1991. EMBO J. 10, 4351.
Filley, E.A., and Rook, G.A. 1991. Infect. Immun.59, 2567.
Landis, R.C., et al. 1991. J. Immunol.146, 128.
Schweighoffer, T., et al. 1991. Histochemistry96, 93.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 1 Oral - Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
June Bryan de la Peña et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 41(37), 7712-7726 (2021-07-31)
Injury responses require communication between different cell types in the skin. Sensory neurons contribute to inflammation and can secrete signaling molecules that affect non-neuronal cells. Despite the pervasive role of translational regulation in nociception, the contribution of activity-dependent protein synthesis
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门