推荐产品
形狀
powder
包裝
pkg of 1 × 5 mg (870792P-5mg)
製造商/商標名
Avanti Research™ - A Croda Brand 870792P
脂質類型
bioactive lipids
sphingolipids
運輸包裝
dry ice
儲存溫度
−20°C
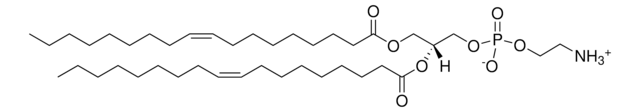
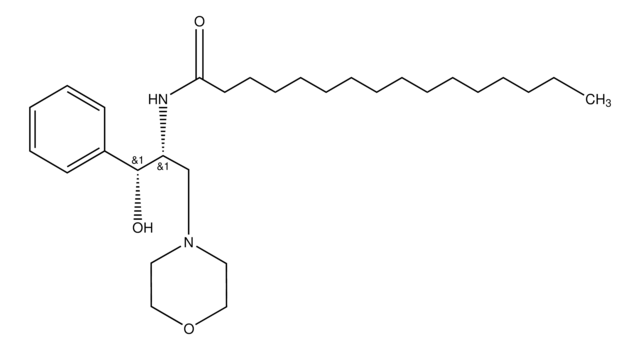
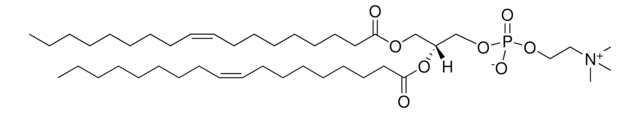
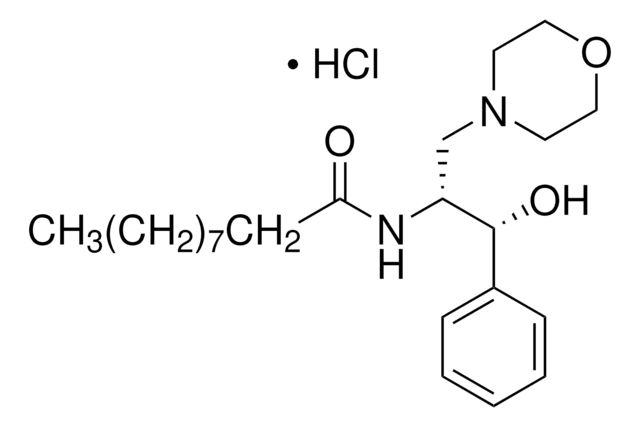
SMILES 字串
O[C@H](C1=CC=CC=C1)[C@H](NC(CCCCCCCCCCCCCCC)=O)CN2CCOCC2
InChI 密鑰
OFBANDBMHLEMFA-XRKRLSELSA-N
應用
D-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (D-threo-PPMP) has been used as an internal standard for the generation of standard curve in high performance liquid chromatography.
生化/生理作用
D-threo-PPMP, also known as 1R,2R-(+)-1-phenyl-2-palmitoylamino-3-N-morpholine-1-propanol, is a bioactive sphingolipid. It plays a vital role in regulation of ceramide metabolism. D-threo-PPMP influences cytokinesis failure and glycosylation by inhibiting glucosyl ceramide synthase (GCS). D-threo-PPMP also stops acylation reaction by inhibiting the activity of 1-O-acylceramide synthase (1-O-ACS).
包裝
5 mL Amber Glass Screw Cap Vial (870792P-5mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
G E Atilla-Gokcumen et al.
Journal of the American Chemical Society, 133(26), 10010-10013 (2011-06-15)
Although cells undergo dramatic shape changes during cytokinesis, the role of the plasma membrane and lipids is poorly understood. We report that inactivation of glucosyl ceramide synthase (GCS), either by RNAi or with the small molecule PPMP, causes failure of
P H O'Donnell et al.
Leukemia, 16(5), 902-910 (2002-05-03)
The retinoid, N-(4-hydroxyphenyl)retinamide (4-HPR), mediates p53-independent cytotoxicity and can increase reactive oxygen species and ceramide in solid tumor cell lines. We determined changes in ceramide and cytotoxicity upon treatment with 4-HPR (3-12 microM) in six human acute lymphoblastic leukemia (ALL)
E I de Chaves et al.
The Journal of biological chemistry, 272(5), 3028-3035 (1997-01-31)
Sphingolipids are abundant constituents of neuronal membranes and have been implicated in intracellular signaling. We show that two analogs of glycosphingolipid biosynthetic intermediates, fumonisin B1 (which inhibits dihydroceramide synthesis) and DL-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP) (which inhibits glucosylceramide synthesis) decrease glycosphingolipid synthesis in
Valérie Gouazé et al.
Cancer research, 65(9), 3861-3867 (2005-05-04)
Overexpression of glucosylceramide synthase (GCS), a pivotal enzyme in glycolipid biosynthesis, contributes to cancer cell resistance to chemotherapy. We previously showed that transfection of doxorubicin-resistant MCF-7-AdrR cells with GCS antisense restored cell sensitivity to doxorubicin and greatly enhanced sensitivity to
B J Maurer et al.
Journal of the National Cancer Institute, 92(23), 1897-1909 (2000-12-07)
We previously reported that N-(4-hydroxyphenyl)retinamide (4-HPR, fenretinide) treatment caused large increases of ceramide levels in neuroblastoma cell lines and induced cell death by a combination of apoptosis and necrosis through p53 (also known as TP53)-independent and caspase-independent pathways. Our goal
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门