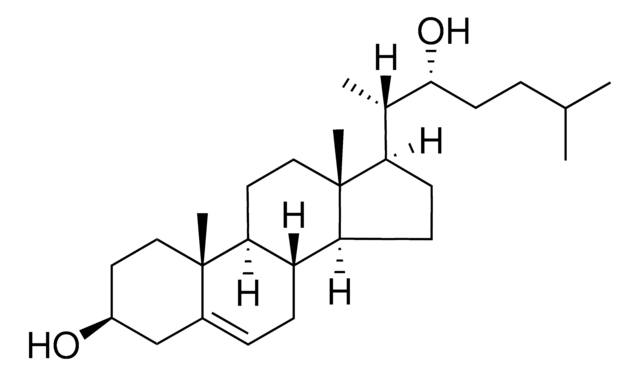
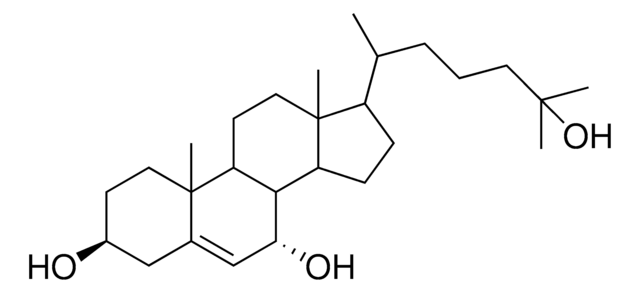
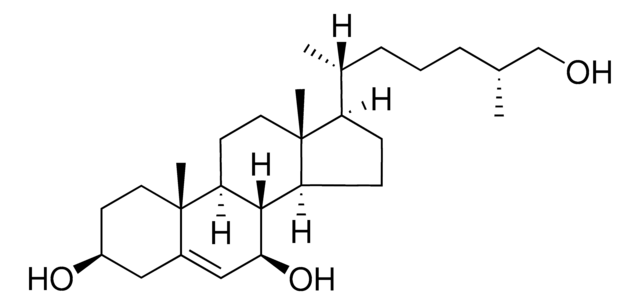
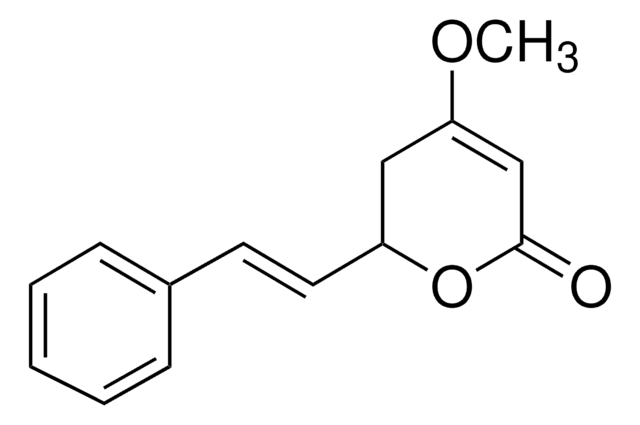
推荐产品
化驗
>99% (TLC)
形狀
powder
包裝
pkg of 1 × 1 mg (700022P-1mg)
製造商/商標名
Avanti Research™ - A Croda Brand
運輸包裝
dry ice
儲存溫度
−20°C
一般說明
7-keto-27-hydroxycholesterol interconversion to 7β,(25R)26-dihydroxycholesterol (7β,26-diHC) is catalyzed by hydroxysteroid 11-β dehydrogenase. It is also formed by the oxidation of 7-oxocholesterol (7-OC) in the presence of enzyme by sterol 26-hydroxylase (CYP27A1).
應用
7-keto-27-hydroxycholesterol has been used as an oxysterol compound to study its interaction with smoothened (SMO) protein, as a substrate to 11β-hydroxysteroid dehydrogenases (11β-HSDs) for kinetic measurement studies,
生化/生理作用
7-keto-27-hydroxycholesterol (7-OC) acts as an agonist for the smoothened (SMO) protein of Hedgehog (Hh) signalling pathway, which is vital for proper cell differentiation in embryonic tissue. It elicits strong affinity to SMO compared to 7β,(25R)26-dihydroxycholesterol (7β,26-diHC). 7-OC is reduced to 7β,(25R)26-dihydroxycholesterol (7β,26-diHC) by reactive oxygen species (ROS).
包裝
5 mL Amber Glass Screw Cap Vial (700022P-1mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
儲存類別代碼
11 - Combustible Solids
Katharina R Beck et al.
Journal of lipid research, 60(9), 1535-1546 (2019-07-06)
Oxysterols previously were considered intermediates of bile acid and steroid hormone biosynthetic pathways. However, recent research has emphasized the roles of oxysterols in essential physiologic processes and in various diseases. Despite these discoveries, the metabolic pathways leading to the different
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门