推荐产品
生物源
synthetic
法律遵循
FDA 21 CFR 117
化驗
≥98%
光學活性
[α]21/D −19.7°, c = 4 in H2O
mp
88-92 °C (lit.)
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
感官的
odorless
SMILES 字串
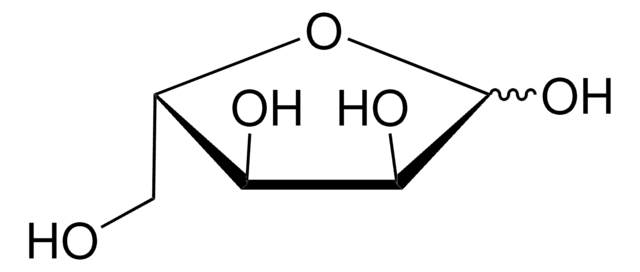
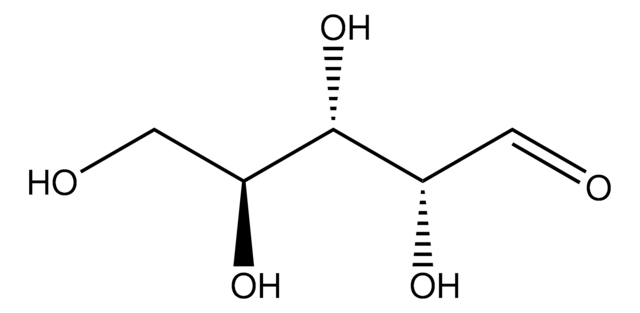
OC[C@@H](O)[C@@H](O)[C@@H](O)C([H])=O
InChI
1S/C5H10O5/c6-1-3(8)5(10)4(9)2-7/h1,3-5,7-10H,2H2/t3-,4+,5-/m0/s1
InChI 密鑰
PYMYPHUHKUWMLA-LMVFSUKVSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
D-(−)-核糖已在体外研究中研究了非酶促核糖在修饰骨胶原蛋白导致骨脆性中的作用。
應用
- Protective effect of thymoquinone on glycation of human myoglobin induced by d-ribose.: This study investigates the protective effects of thymoquinone against d-ribose-induced glycation in human myoglobin, highlighting potential therapeutic applications in preventing protein glycation (Liu et al., 2023).
免責聲明
用于研发&或非欧盟(EU)食品用途。不用于零售。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
D-ribose and deoxy-D-ribose induce apoptosis in human quiescent peripheral blood mononuclear cells.
Barbieri D, et al.
Biochemical and Biophysical Research Communications, 201(3), 1109-1116 (1994)
Alice Pavlowsky et al.
Current biology : CB, 28(11), 1783-1793 (2018-05-22)
Memory consolidation is a crucial step for long-term memory (LTM) storage. However, we still lack a clear picture of how memory consolidation is regulated at the neuronal circuit level. Here, we took advantage of the well-described anatomy of the Drosophila
Thomas L Willett et al.
Bone, 52(2), 611-622 (2012-11-28)
Non-enzymatic glycation (NEG) and advanced glycation endproducts (AGEs) may contribute to bone fragility in various diseases, ageing, and other conditions by modifying bone collagen and causing degraded mechanical properties. In this study, we sought to further understand how collagen modification
Lusani Norah Vhangani et al.
Food chemistry, 137(1-4), 92-98 (2012-12-04)
Maillard reaction products (MRPs) were prepared from aqueous ribose-lysine (RL) and fructose-lysine (FL) model systems at pH 9, heated at 60, 80 and 120 °C for 15, 60 and 120 min. Browning intensity (BI) and pH reduction were monitored throughout
Raman K Sharma et al.
Bioorganic & medicinal chemistry, 20(23), 6821-6830 (2012-10-27)
A series of peracetylated O-aryl α,β-d-ribofuranosides have been synthesized and an efficient biocatalytic methodology has been developed for the separation of their anomers which was otherwise almost impossible by column chromatographic or other techniques. The incubation of 2,3,5-tri-O-acetyl-1-O-aryl-α,β-d-ribofuranoside with Lipozyme®
实验方案
Separation of Ribose, United States Pharmacopeia (USP) Reference Standard; Xylitol, analytical standard; Mannose, United States Pharmacopeia (USP) Reference Standard
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门