推荐产品
品質等級
化驗
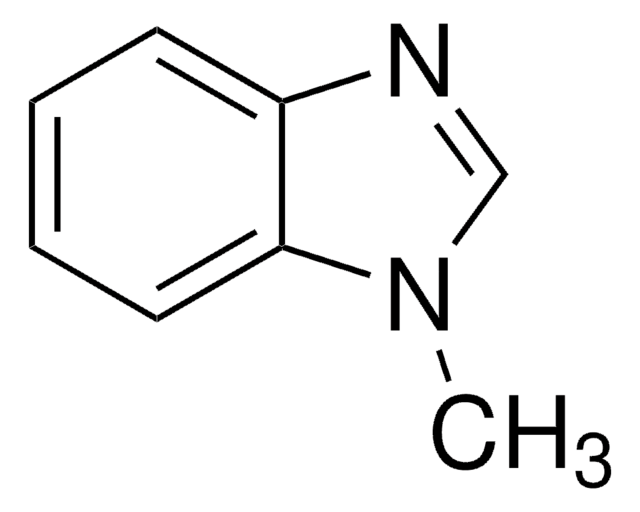
98%
mp
175-177 °C (lit.)
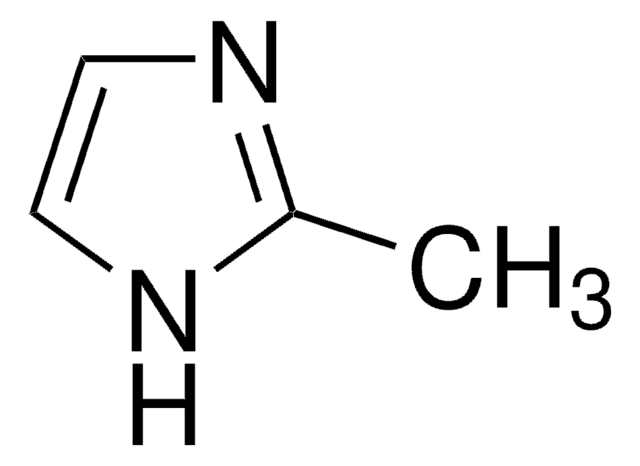
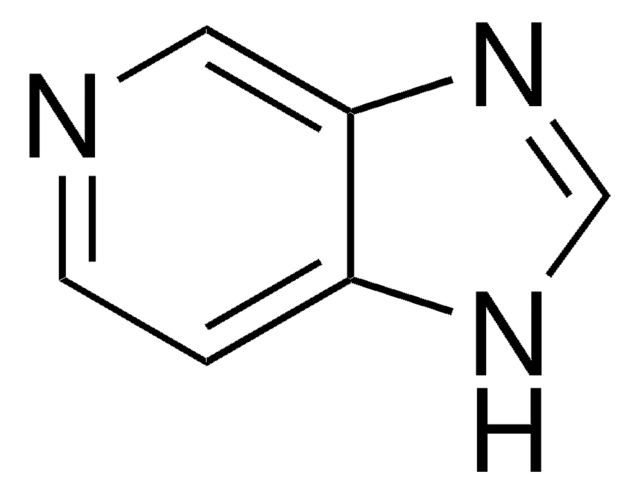
SMILES 字串
Cc1nc2ccccc2[nH]1
InChI
1S/C8H8N2/c1-6-9-7-4-2-3-5-8(7)10-6/h2-5H,1H3,(H,9,10)
InChI 密鑰
LDZYRENCLPUXAX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- 2-甲基苯并咪唑是一种重要的药效团,广泛用于药物化学中,可用于合成各种抗菌和抗真菌剂。
- 可用作合成取代的苯并咪唑[1,2-a]喹诺酮类物质的关键前体。
- 它可用于合成可逆的固-液相转变配位聚合物晶体。
- 2-甲基苯并咪唑还表现出腐蚀抑制作用。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Synthesis and biological evaluation of substituted benzimidazoles.
Shah K, et al.
Journal of the Indian Chemical Society, 93, 1009-1018 (2016)
Deidré van den Berg et al.
Bioorganic & medicinal chemistry, 15(11), 3692-3702 (2007-04-10)
We have recently reported that a series of (E)-8-styrylcaffeines and (E)-2-styrylbenzimidazoles are moderate to very potent competitive inhibitors of monoamine oxidase B (MAO-B). The most potent member of the series was found to be (E)-8-(3-chlorostyryl)caffeine (CSC) with an enzyme-inhibitor dissociation
Reversible solid-to-liquid phase transition of coordination polymer crystals.
Umeyama D, et al.
Journal of the American Chemical Society, 137(2), 864-870 (2015)
Synthesis and Antimicrobial Activity of Some Benzimidazole and 2-Methylbenzimidazole Derivatives.
Jain P and Tiwari M
Asian Journal of Chemistry, 29(4), 838-838 (2017)
Novel strategy for synthesis of substituted benzimidazo [1, 2-a] quinolines.
Kato J Y, et al.
Organic Letters, 15(14), 3794-3797 (2013)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门