推荐产品
品質等級
化驗
97%
mp
246-248 °C (lit.)
SMILES 字串
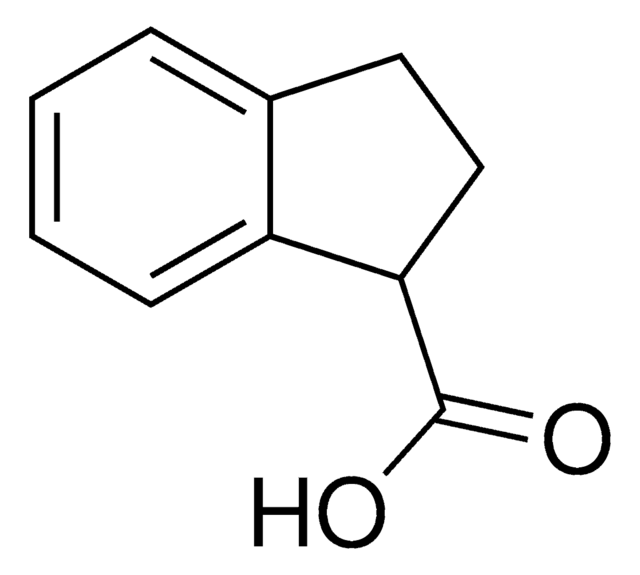
OC(=O)c1cccc-2c1Cc3ccccc-23
InChI
1S/C14H10O2/c15-14(16)12-7-3-6-11-10-5-2-1-4-9(10)8-13(11)12/h1-7H,8H2,(H,15,16)
InChI 密鑰
HTPXFGUCAUTOEL-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
A C Blackburn et al.
Acta crystallographica. Section C, Crystal structure communications, 52 ( Pt 4), 907-910 (1996-04-15)
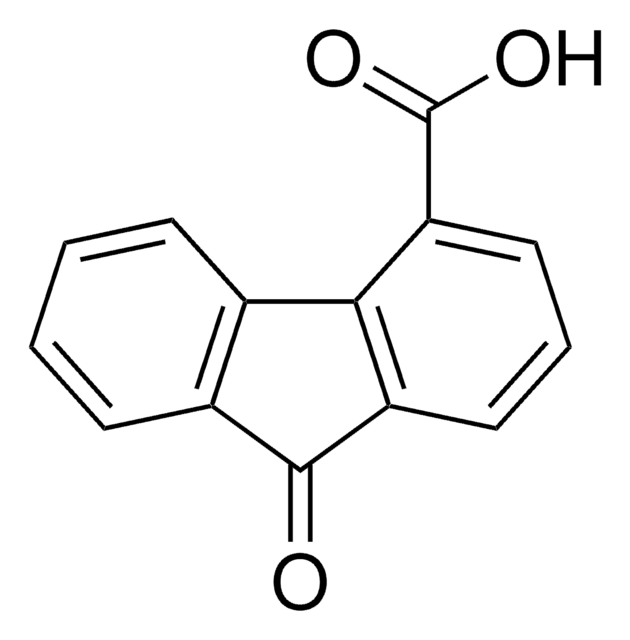
In fluorene-1-carboxylic acid, C14H10O2, the sole hydrogen bond is of the cyclic dimer type about a center of symmetry. The carboxyl H atom is ordered. Distances in the fluorene core are very similar to those in fluorene itself; the fluorene
William Kemnitzer et al.
Bioorganic & medicinal chemistry letters, 20(3), 1288-1292 (2009-12-26)
As a continuation of our studies of apoptosis inducing 9-oxo-9H-fluorene-1-carboxamides as potential anticancer agents, we explored modification of the 9-oxo-9H-fluorene ring. SAR studies showed that most changes to the 9-oxo-9H-fluorene ring were not well tolerated, except the 9H-fluorene (2b) and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门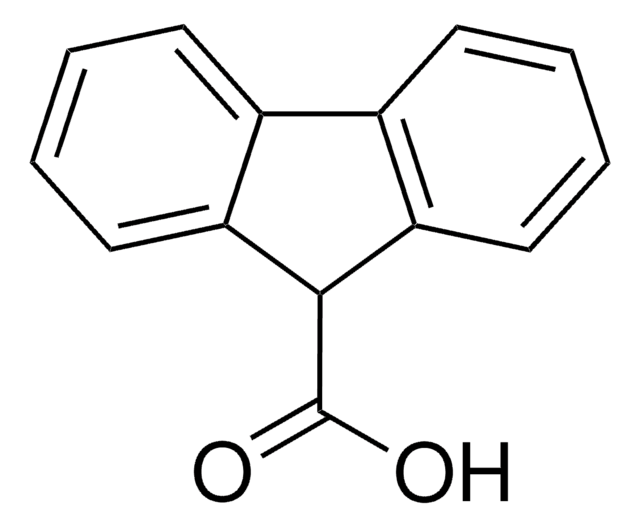
![[Ru(phen)3]Cl2 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/955/596/46f63eaa-39f8-4058-847d-cef0862ada92/640/46f63eaa-39f8-4058-847d-cef0862ada92.png)