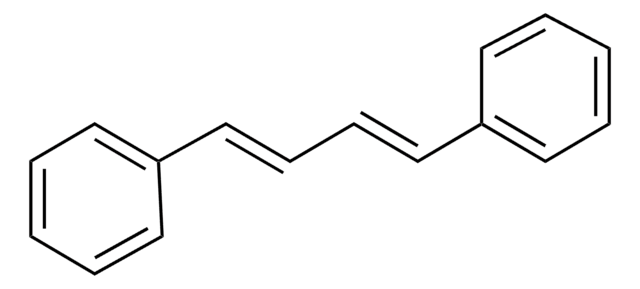
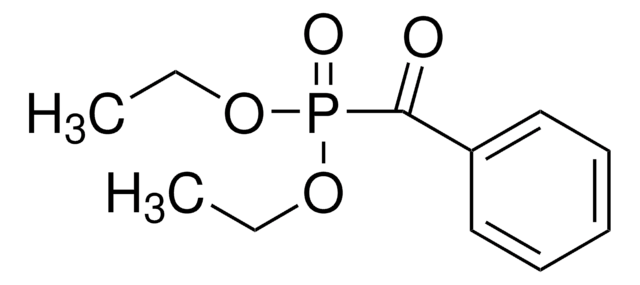
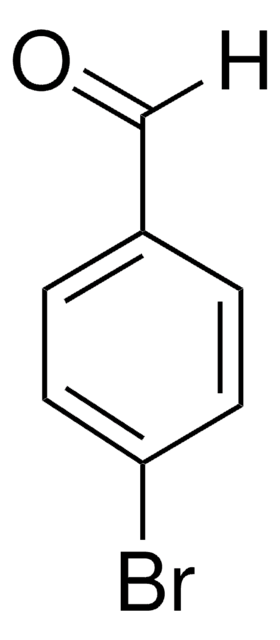
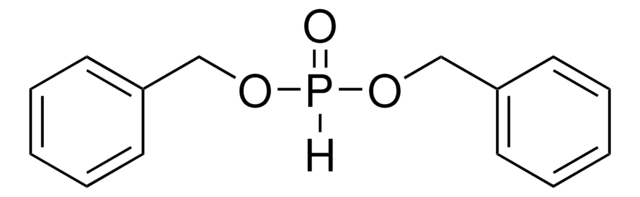
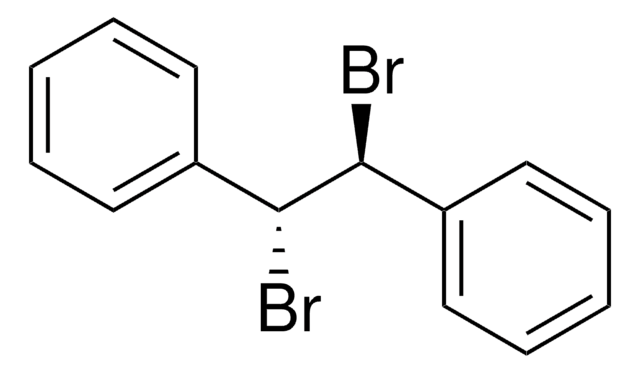
推荐产品
化驗
99%
形狀
liquid
反應適用性
reaction type: C-C Bond Formation
折射率
n20/D 1.497 (lit.)
bp
106-108 °C/1 mmHg (lit.)
密度
1.095 g/mL at 25 °C (lit.)
SMILES 字串
CCOP(=O)(Cc1ccccc1)OCC
InChI
1S/C11H17O3P/c1-3-13-15(12,14-4-2)10-11-8-6-5-7-9-11/h5-9H,3-4,10H2,1-2H3
InChI 密鑰
AIPRAPZUGUTQKX-UHFFFAOYSA-N
應用
用于合成以下物质的反应物:
用作以下过程的反应物:
- 3,5-二羟基-4-异丙基二苯乙烯(治疗皮肤疾病)
- 通过分子内 Diels-Alder 反应来源的天然细胞毒性海产品
- 邻二醇的柱上氧化和 Horner-Emmons 反应合成的二苯乙烯
- Wnt 通路抑制剂(使用 Wadsworth-Emmons 反应抑制结肠癌)
- 抗恶性疟原虫的抗疟药类似物
用作以下过程的反应物:
- 芳基醚、胺和酰胺的环化
- 研究官能团对线索有机发光二极管性能的影响
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
Ananya Thomas et al.
Polymers, 12(8) (2020-08-17)
As a part of our ongoing investigations on passively fire protecting polymeric materials, we have been employing both reactive and additive routes involving phosphorus-containing compounds. These included inorganic and organic substances, and in the latter case, the phosphorus-bearing groups differed
Jack Saltiel et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 18(9), 2174-2179 (2019-05-28)
cis,trans-1,2-Dideuterio-1,4-diphenyl-1,3-butadiene (ct-DPBd2) was synthesized and its cis-trans photoisomerization in cyclohexane-d12 (C6D12) at room temperature was monitored by 1H NMR spectroscopy. The results reveal formation of only trans,trans-1,2-dideuterio-1,4-diphenyl-1,3-butadiene (tt-DPBd2). The failure to detect formation of trans,cis-1,2-dideuterio-1,4-diphenyl-1,3-butadiene (tc-DPBd2) eliminates the possibility that
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门