推荐产品
蒸汽密度
4.2 (vs air)
品質等級
產品線
ReagentPlus®
化驗
99%
形狀
liquid
自燃溫度
554 °F
包含
≤1000 ppm propylene oxide as stabilizer
expl. lim.
7.3 %
折射率
n20/D 1.469 (lit.)
bp
70-71 °C (lit.)
mp
−119 °C (lit.)
密度
1.398 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
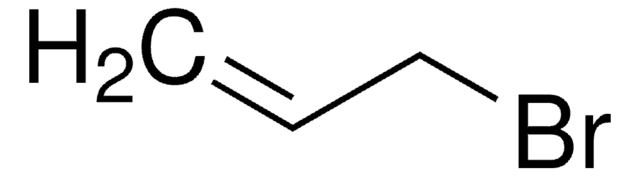
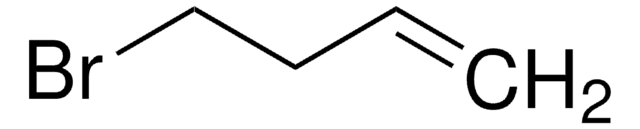
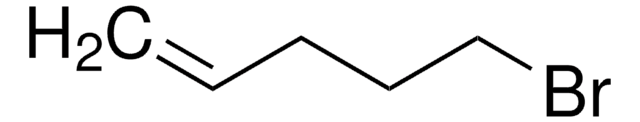
SMILES 字串
BrCC=C
InChI
1S/C3H5Br/c1-2-3-4/h2H,1,3H2
InChI 密鑰
BHELZAPQIKSEDF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
烯丙基溴可作为制备以下物质的反应剂:
- 在路易酸的作用下通过各种腈的 Barbier 型烯丙基化制备烯丙基酮。
- 使用锌粉作为催化剂,与醛类或酮 类反应制备高烯丙醇。
- 通过氢化银团簇还原 C-C 键偶联制备 1,5-己二烯。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Carc. 1B - Eye Dam. 1 - Flam. Liq. 2 - Muta. 1B - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
30.2 °F - closed cup
閃點(°C)
-1 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Facile and efficient synthesis of homoallylic alcohols using allyl bromide and commercial zinc dust
Ranu BC, et al.
Tetrahedron Letters, 36(27), 4885-4888 (1995)
Synthesis of allyl ketone via Lewis acid promoted Barbier-type reaction
Lee Adam S-Y and Lin L-S
Tetrahedron Letters, 41(45), 8803-8806 (2000)
Jing-Mei Huang et al.
Chemical communications (Cambridge, England), (26)(26), 3943-3945 (2009-08-08)
An efficient electrosynthesis of homoallylic alcohols from allylic bromides and aldehydes in aqueous ammonia is achieved in an undivided cell fitted with a pair of zinc electrodes.
Xiaoyu Yan et al.
Chemical communications (Cambridge, England), 46(41), 7801-7803 (2010-09-22)
Reaction of alkynylzirconates with allyl bromides afforded β-allyl-zirconacyclopentadienes with high selectivity in unique reaction site.
José C González-Gómez et al.
The Journal of organic chemistry, 75(18), 6308-6311 (2010-08-21)
The combination of an aldehyde, an allylic bromide, and tert-butanesulfinamide in the presence of indium metal and titanium tetraethoxide allows straightforward access to homoallylamine derivatives in high yields and stereoselectivities. Moreover, the synthetic utility of the enantioenriched homoallylamine derived from
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门